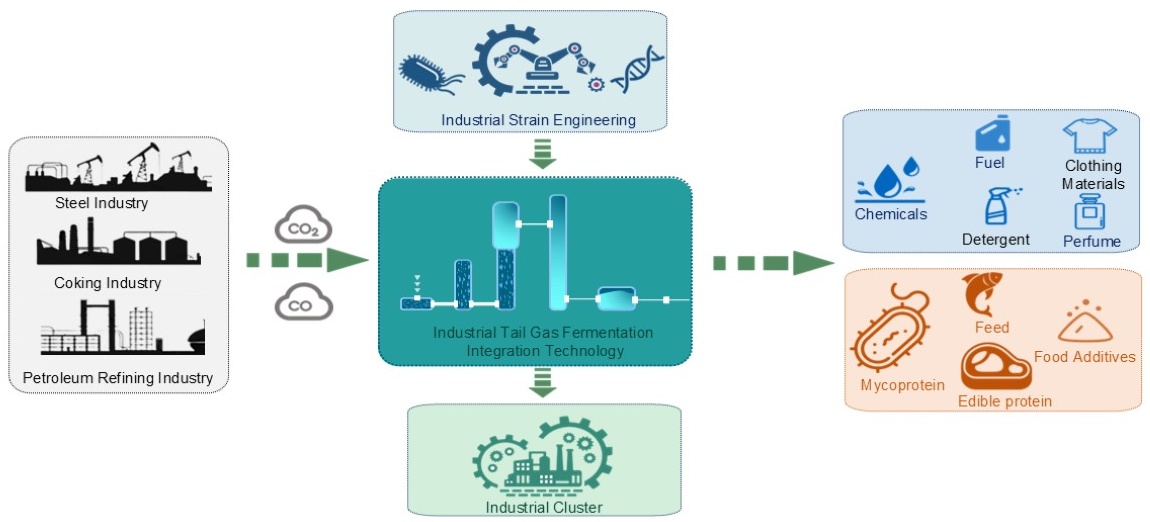
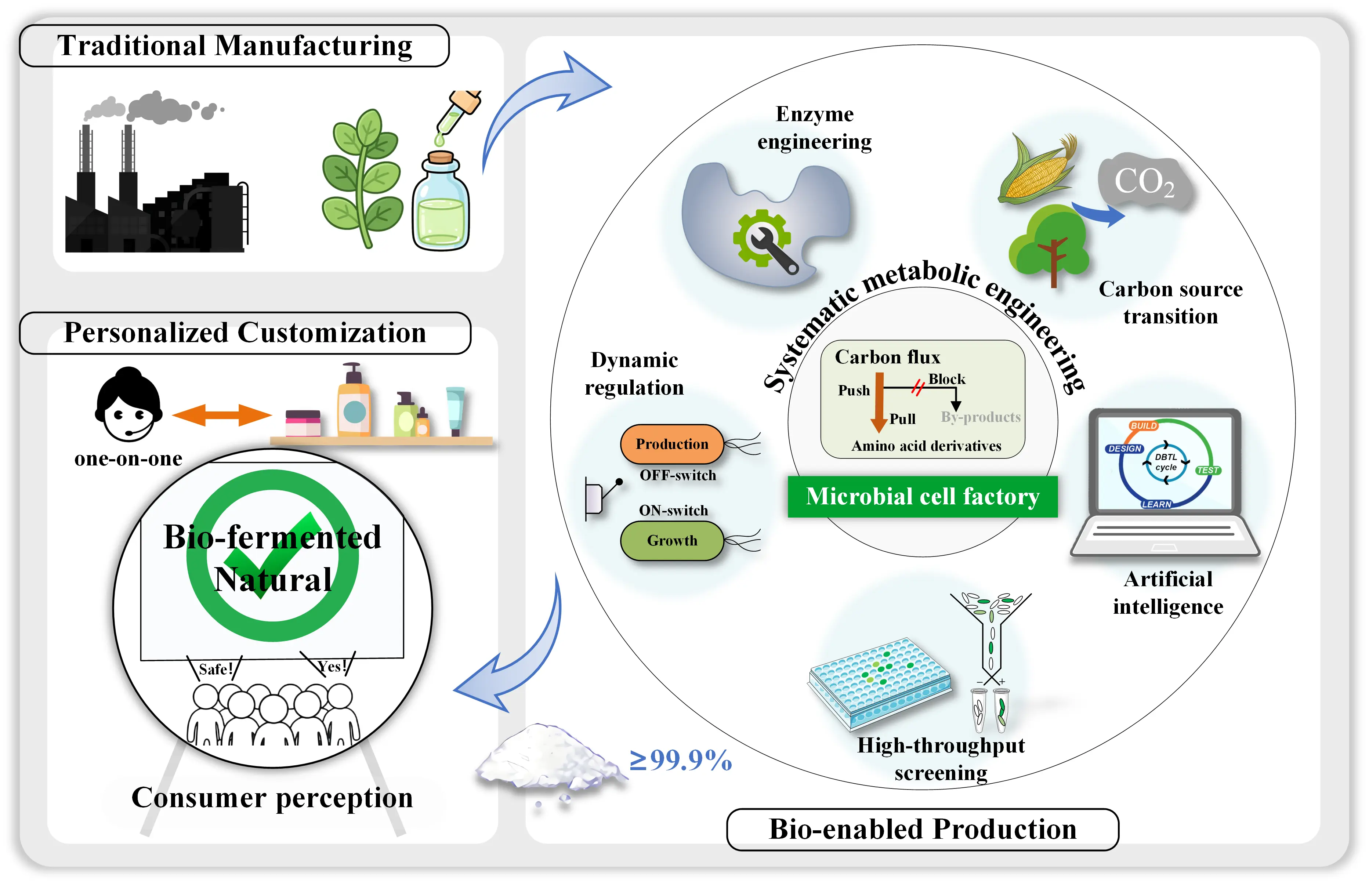
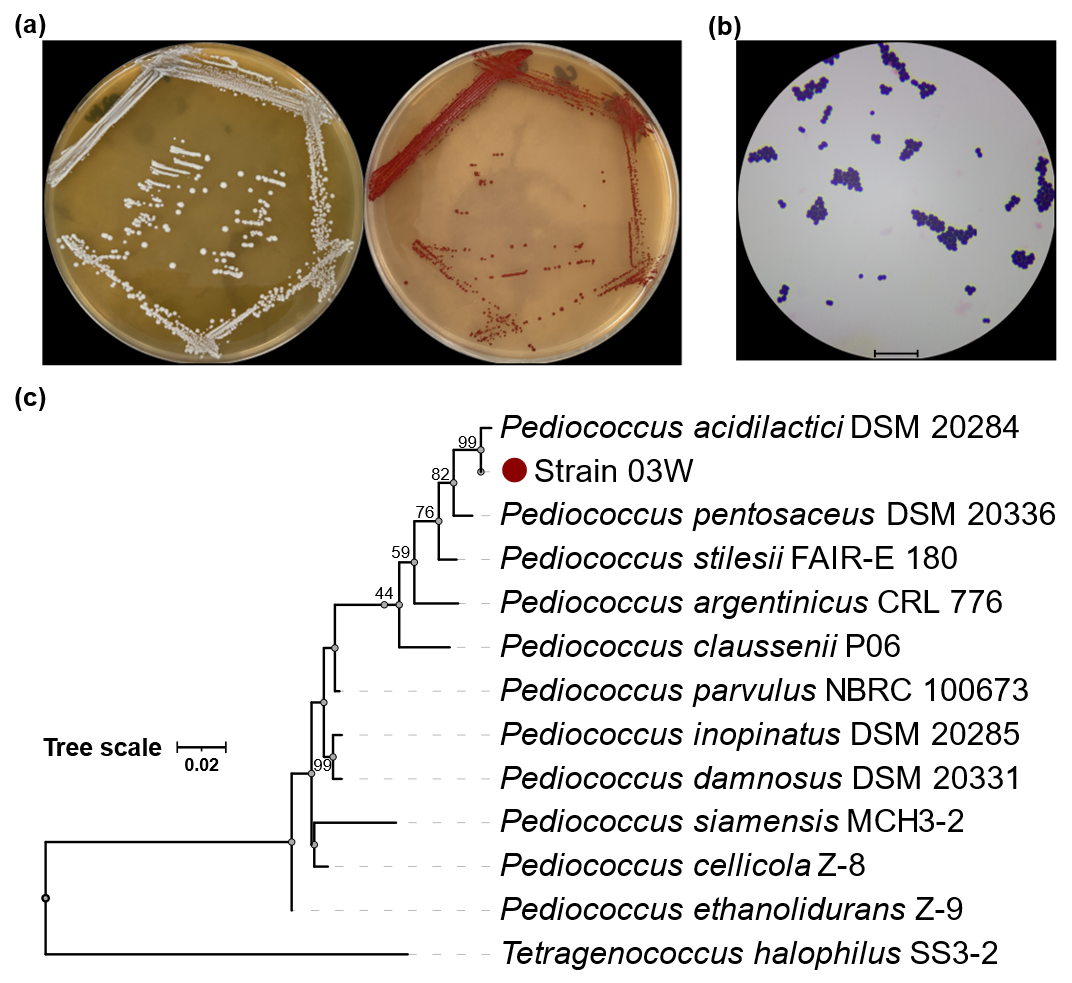
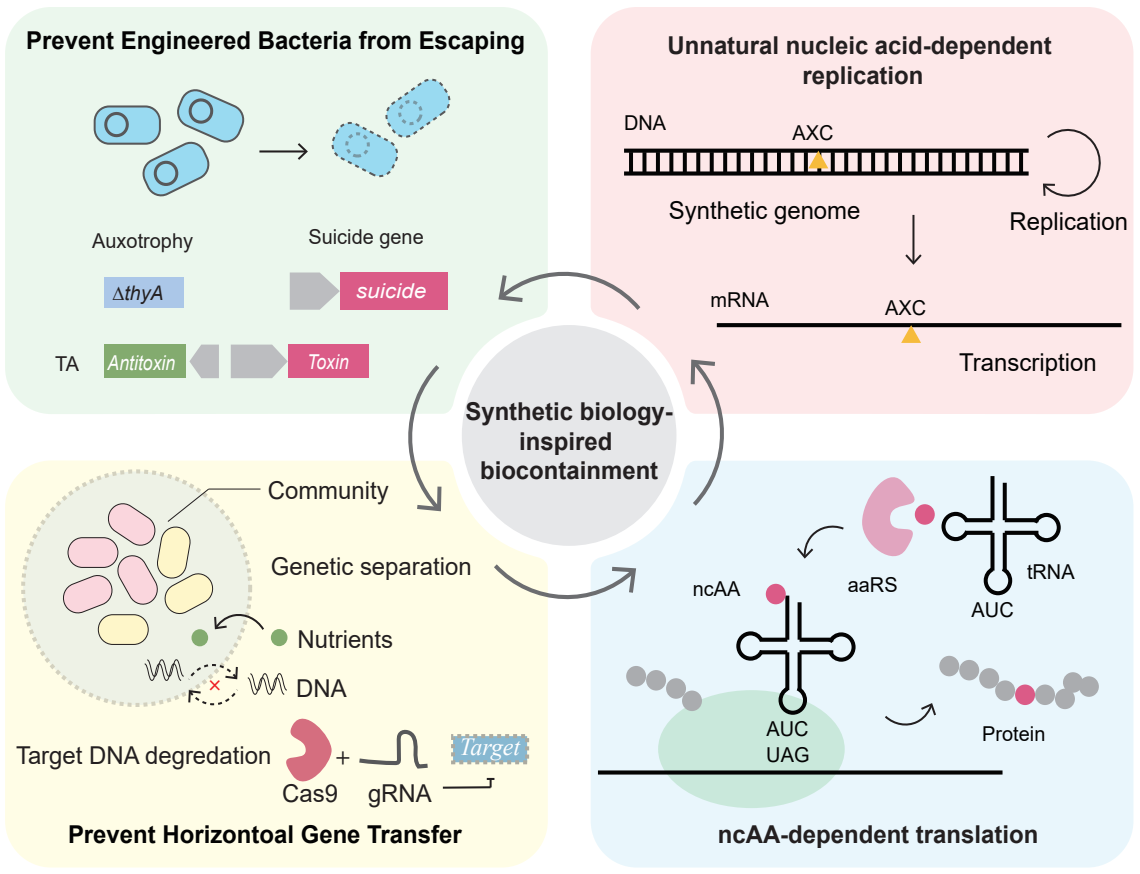
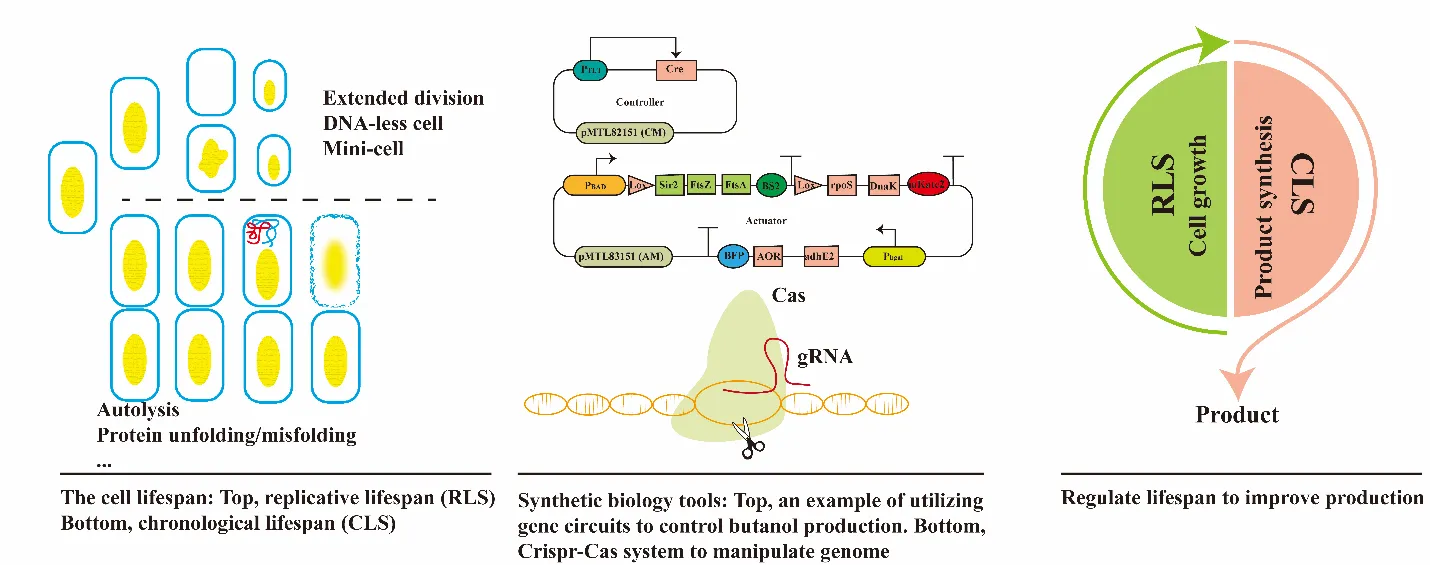
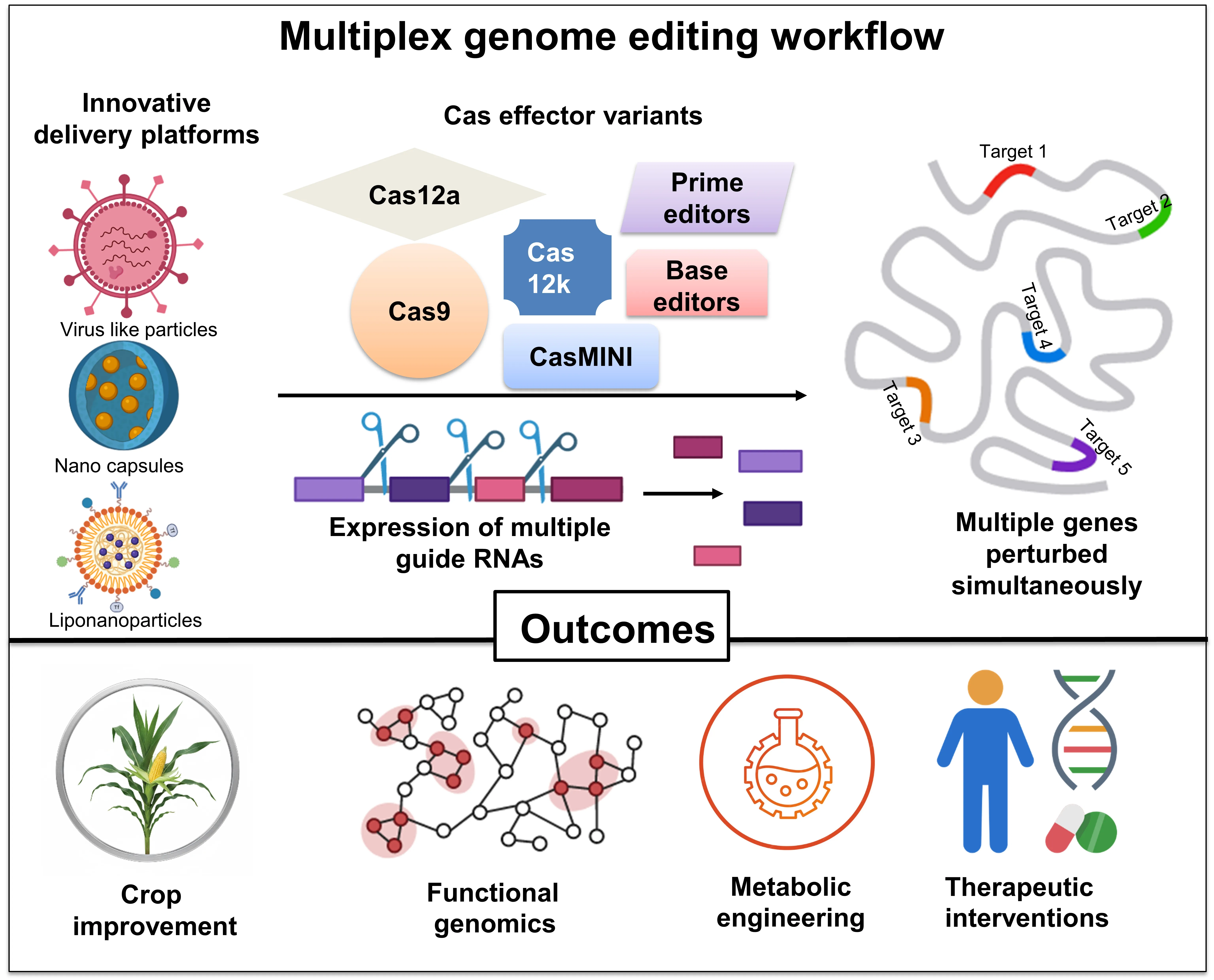
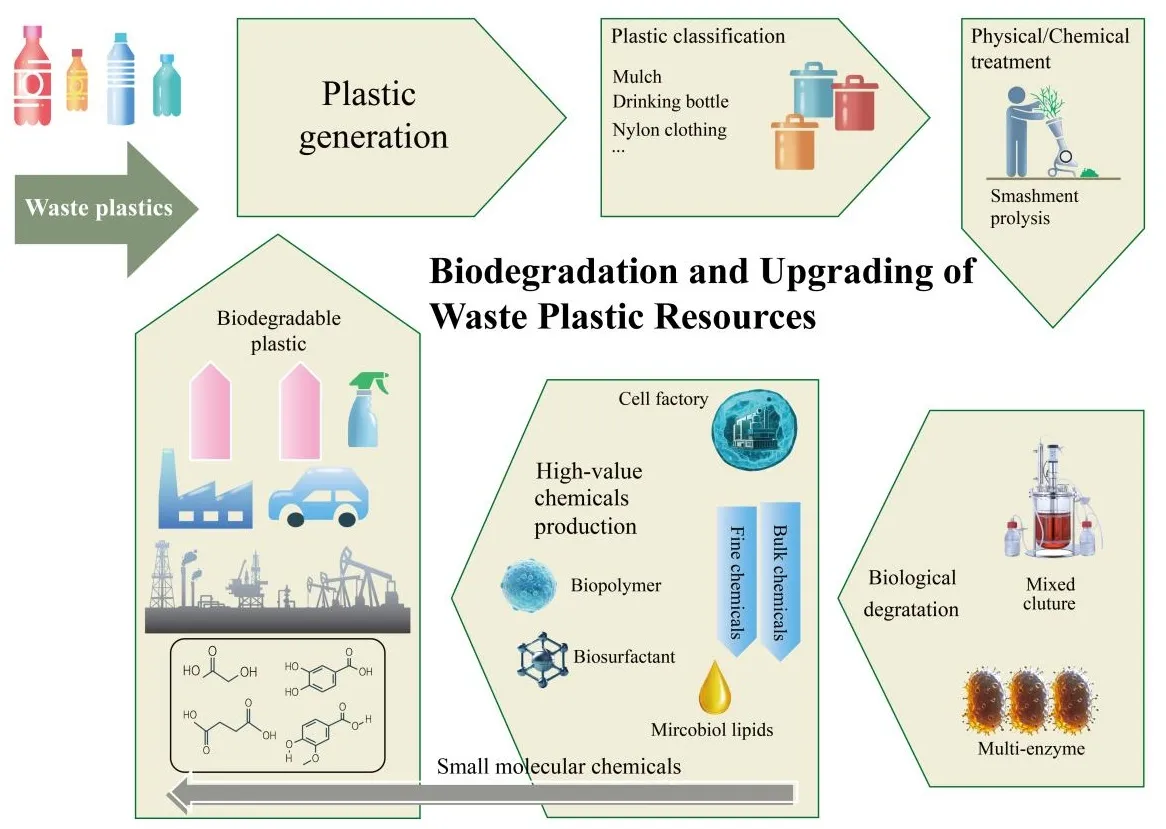
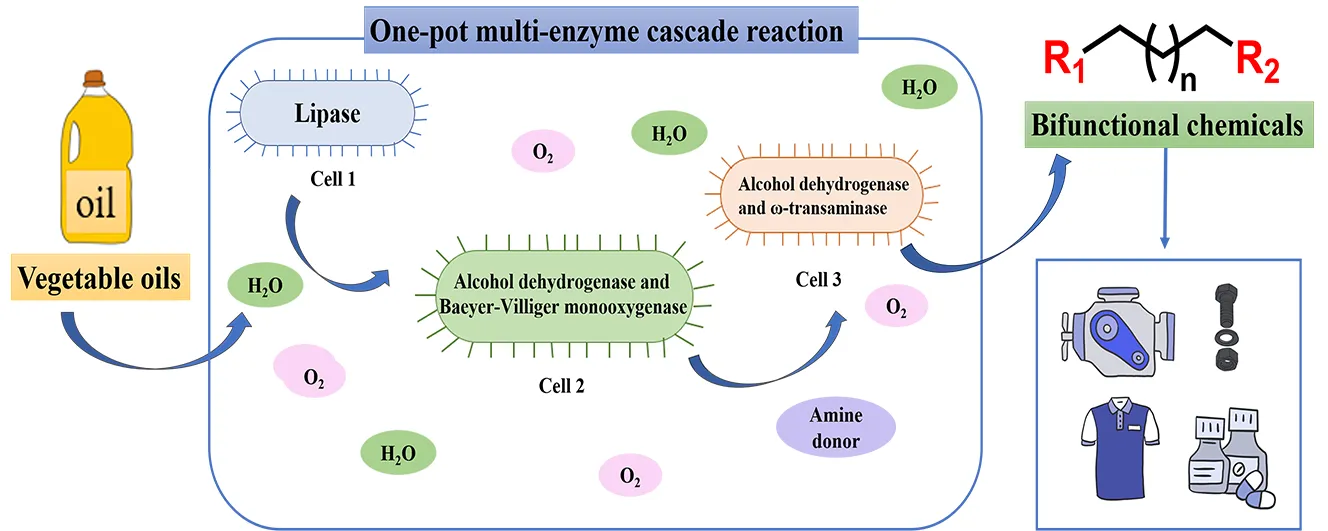
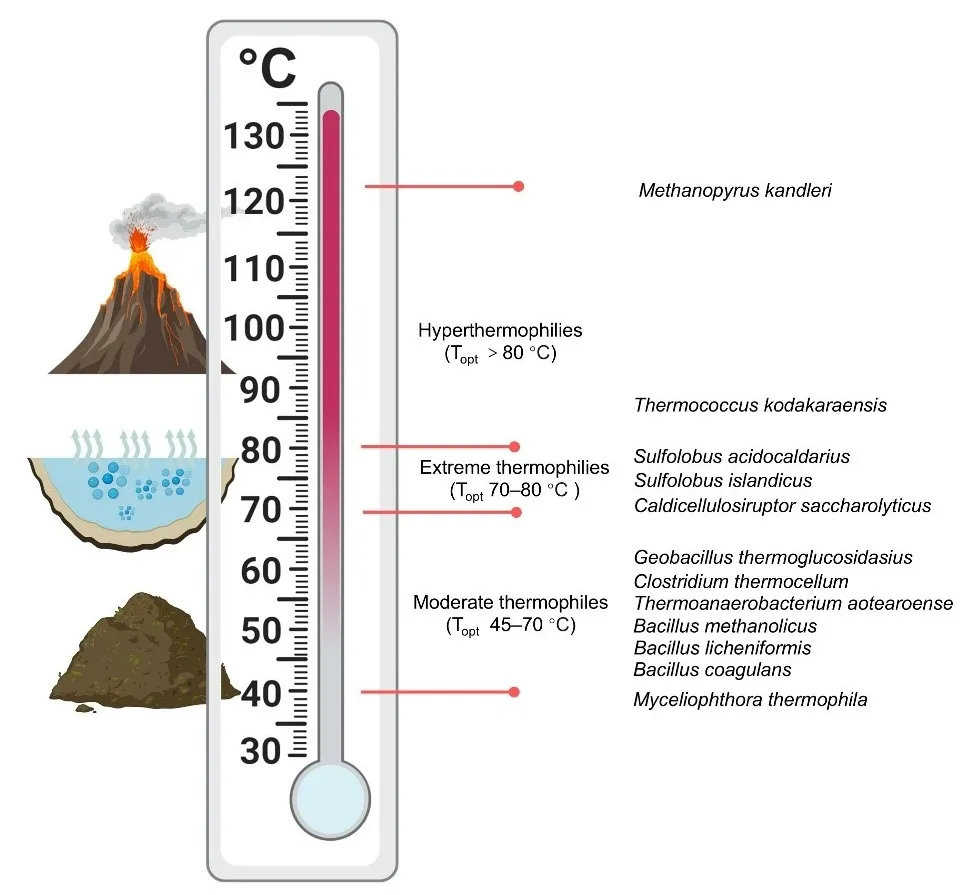
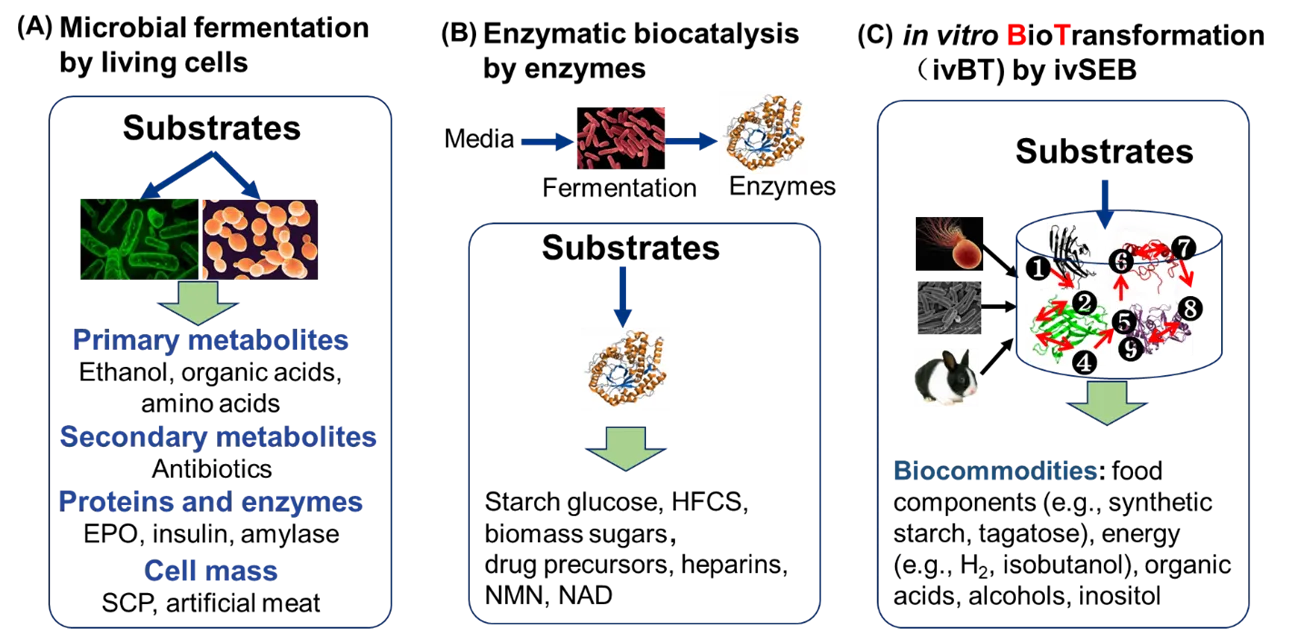
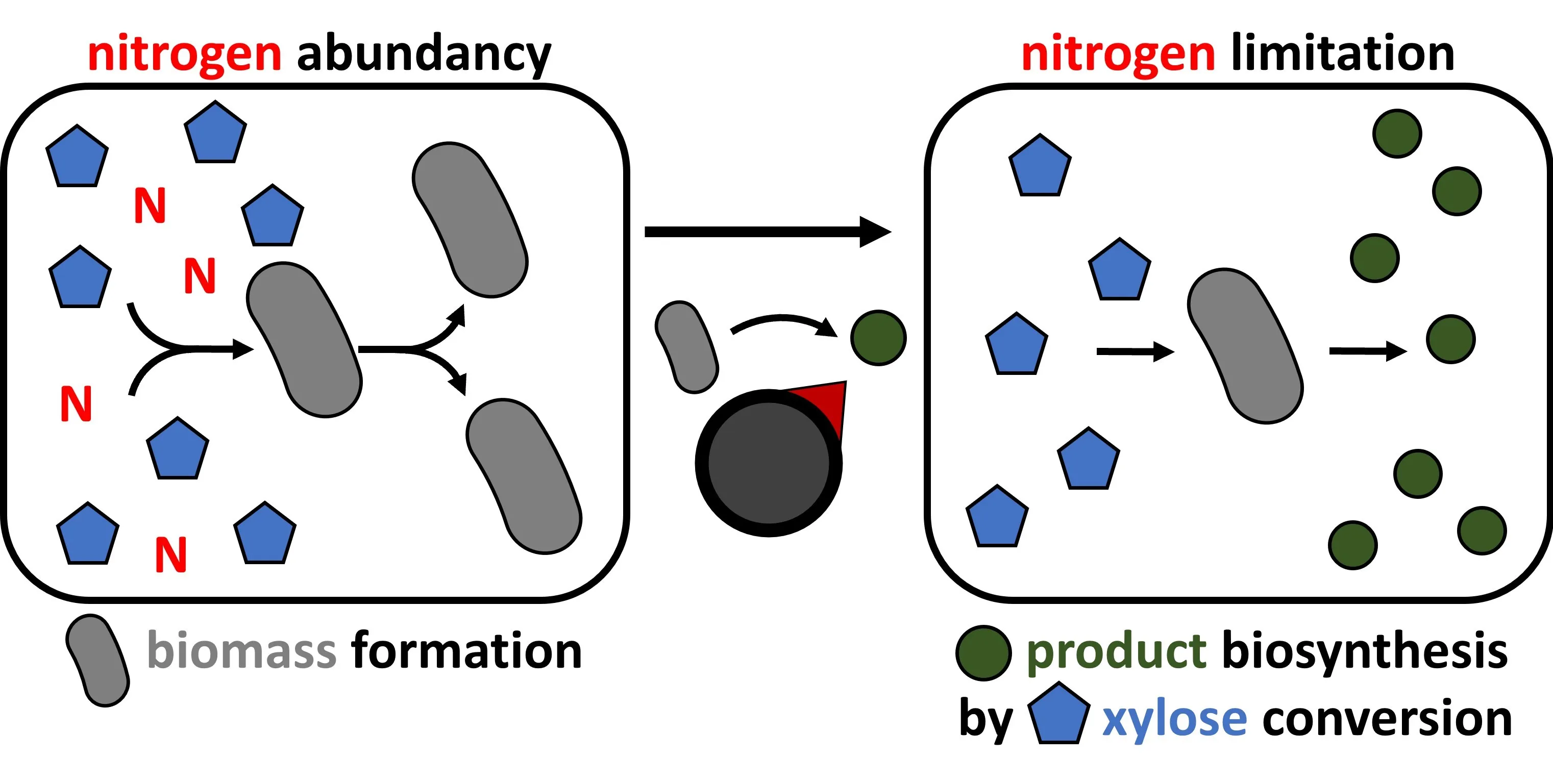
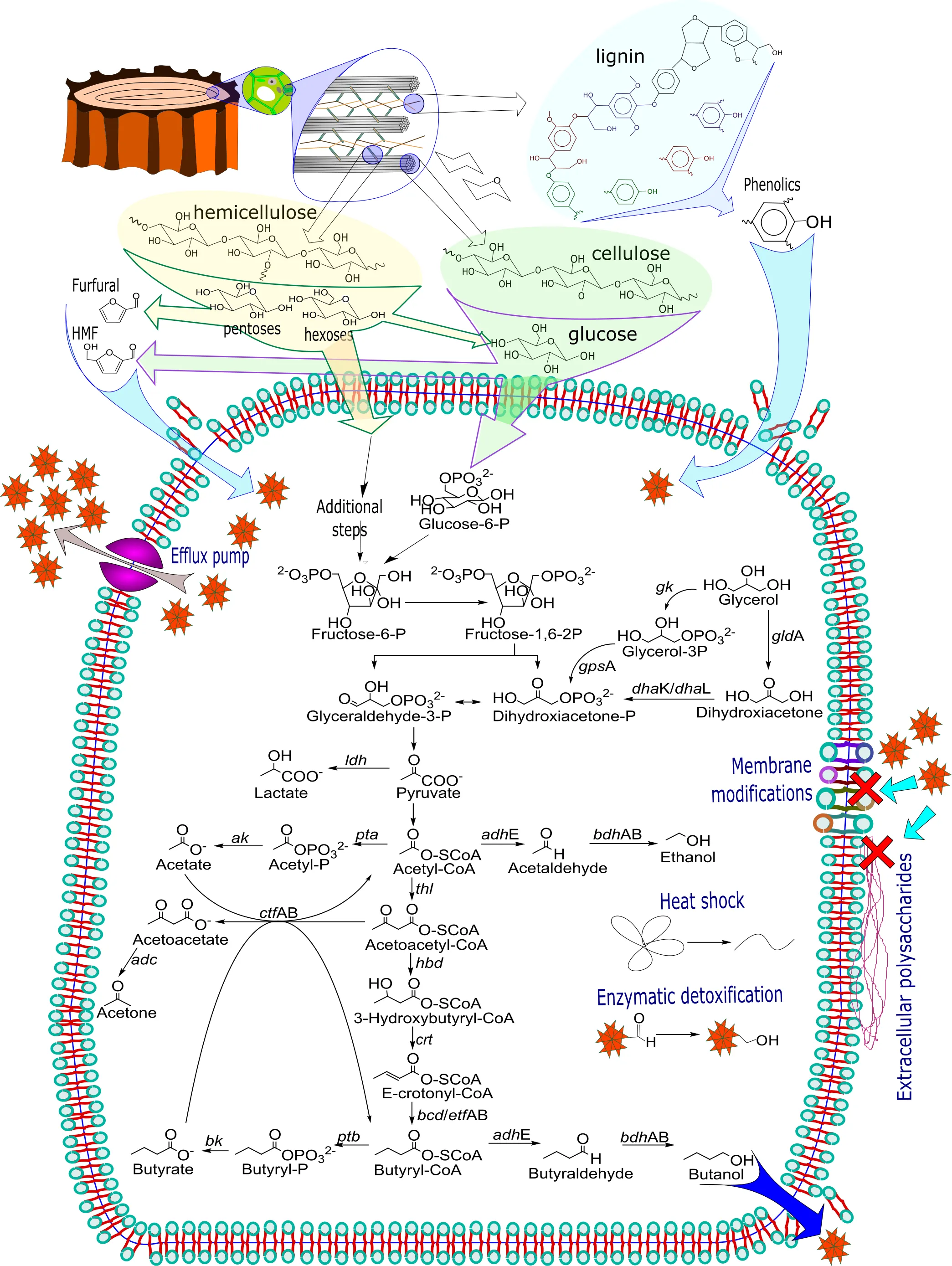
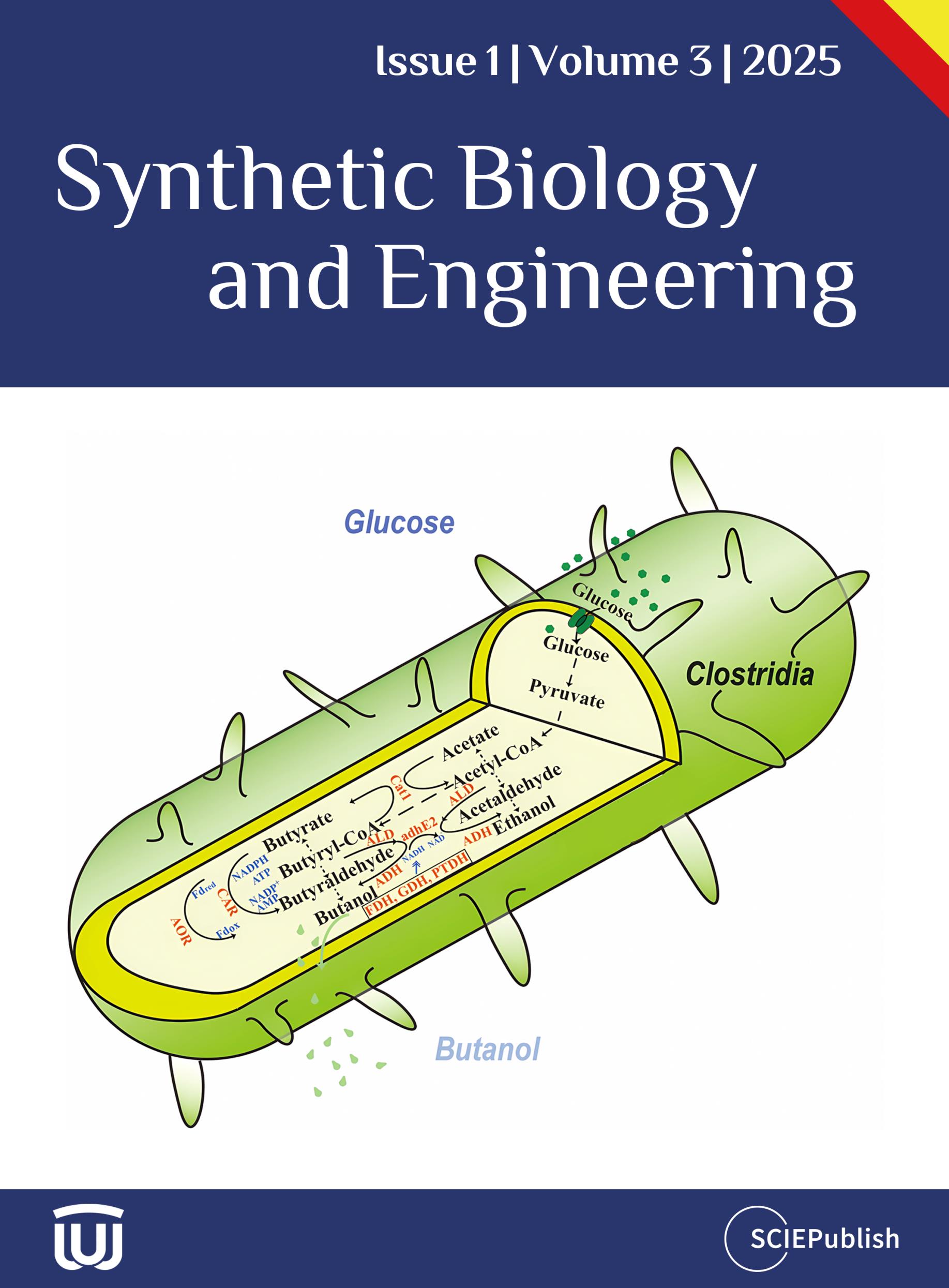
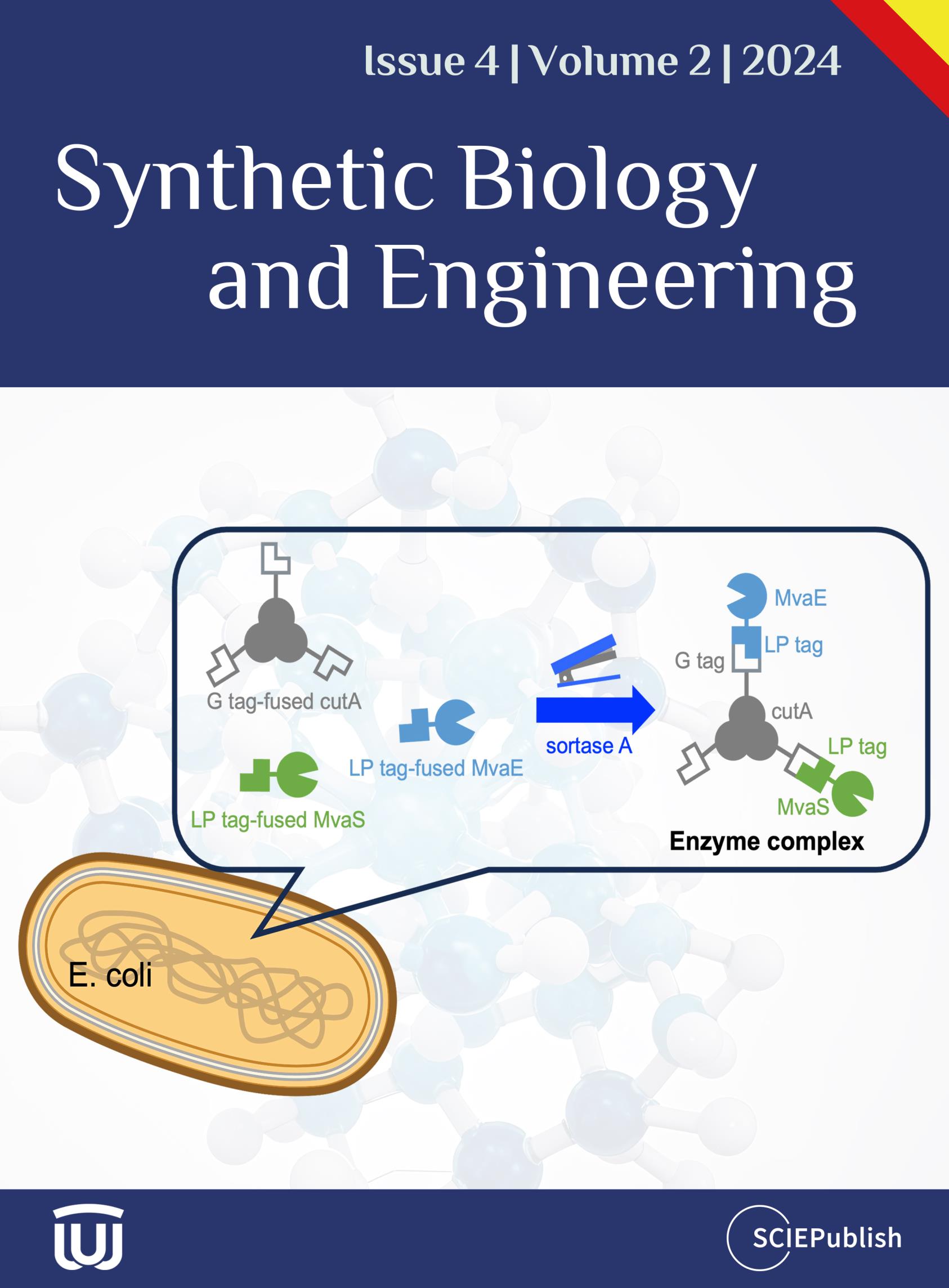
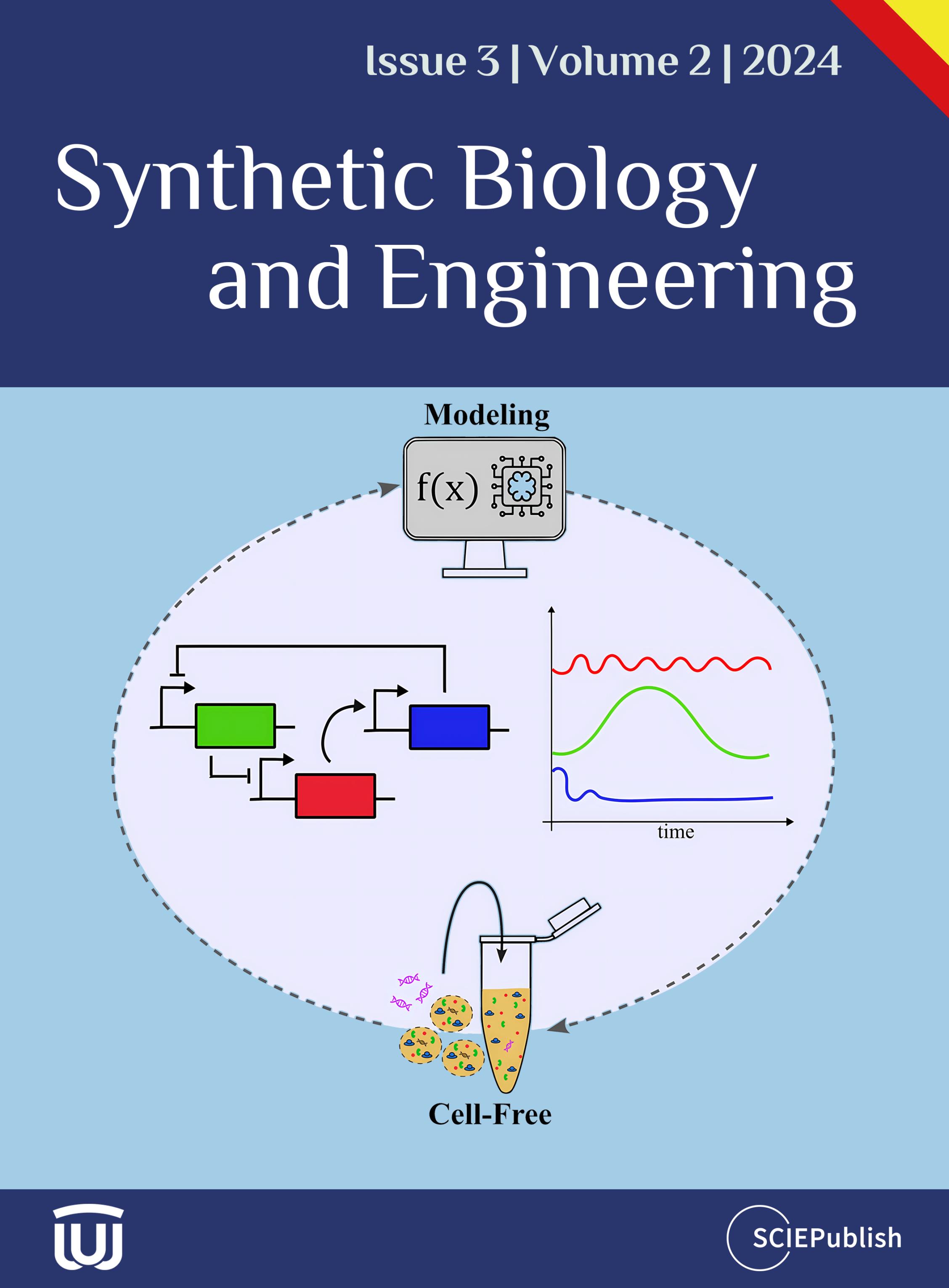
The high molecular
weight, hydrophobicity, and strong chemical bonds of petroleum-based synthetic
plastics make them highly resistant to both abiotic and microbial degradation.
This resistance plays a significant role in the growing problem of “white pollution”
where the accumulation of plastic waste has become a major environmental issue
worldwide. Currently, plastic waste management relies largely on landfill
disposal and incineration, with only about 20% of plastic waste being recycled.
However, both methods create secondary environmental risks, such as
contamination of groundwater, soil, air, and oceans. Therefore, developing a
sustainable and efficient approach for recycling and reusing plastic waste is
essential for tackling plastic pollution and promoting a circular plastic
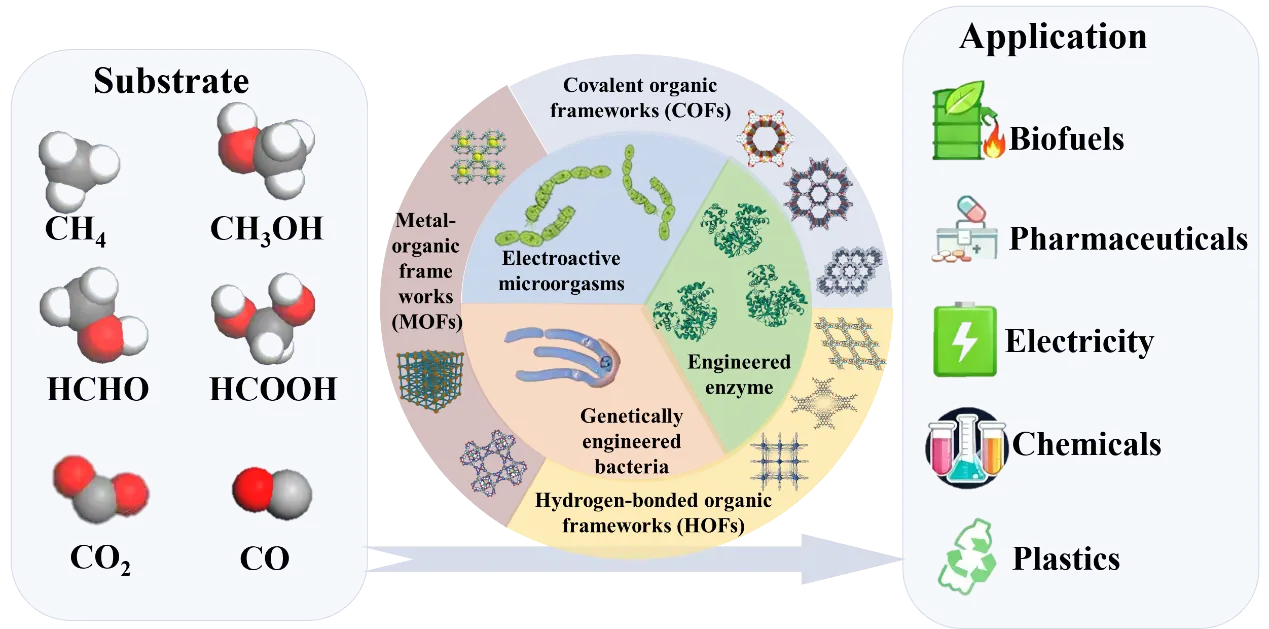
economy. One promising solution involves utilizing microorganisms and enzymes
to break down plastics into oligomers or monomers, which can then be
transformed into valuable chemicals. This method provides a more
environmentally friendly and milder alternative to conventional waste
management techniques. This review explores recent progress in
biodepolymerization and biotransformation processes for plastic waste,
including the identification of plastic-degrading microorganisms and enzymes,
the creation of microbial consortia and enzyme mixtures, an investigation into
the mechanisms of plastic depolymerization, and the conversion of degradation
products into useful materials such as chemicals, energy, and other resources.
Despite these advancements, several challenges remain, such as the limited
availability of effective degradation enzymes, low degradation efficiency, and
difficulties in utilizing the breakdown products. However, emerging
technologies in synthetic biology, such as high-throughput screening,
evolutionary metabolic engineering, and bioinformatics to study catalytic
mechanisms of degradation enzymes, offer promising solutions to address these
issues. By improving enzyme design, optimizing microbial consortia interactions,
and developing efficient metabolic pathways for plastic degradation products,
these innovations could greatly enhance plastic biodegradation. These
advancements hold the potential to provide environmentally sustainable,
economically feasible, and technically viable solutions for promoting a
circular plastic economy, particularly in countries like China.utf-8
 Open Access
Open Access