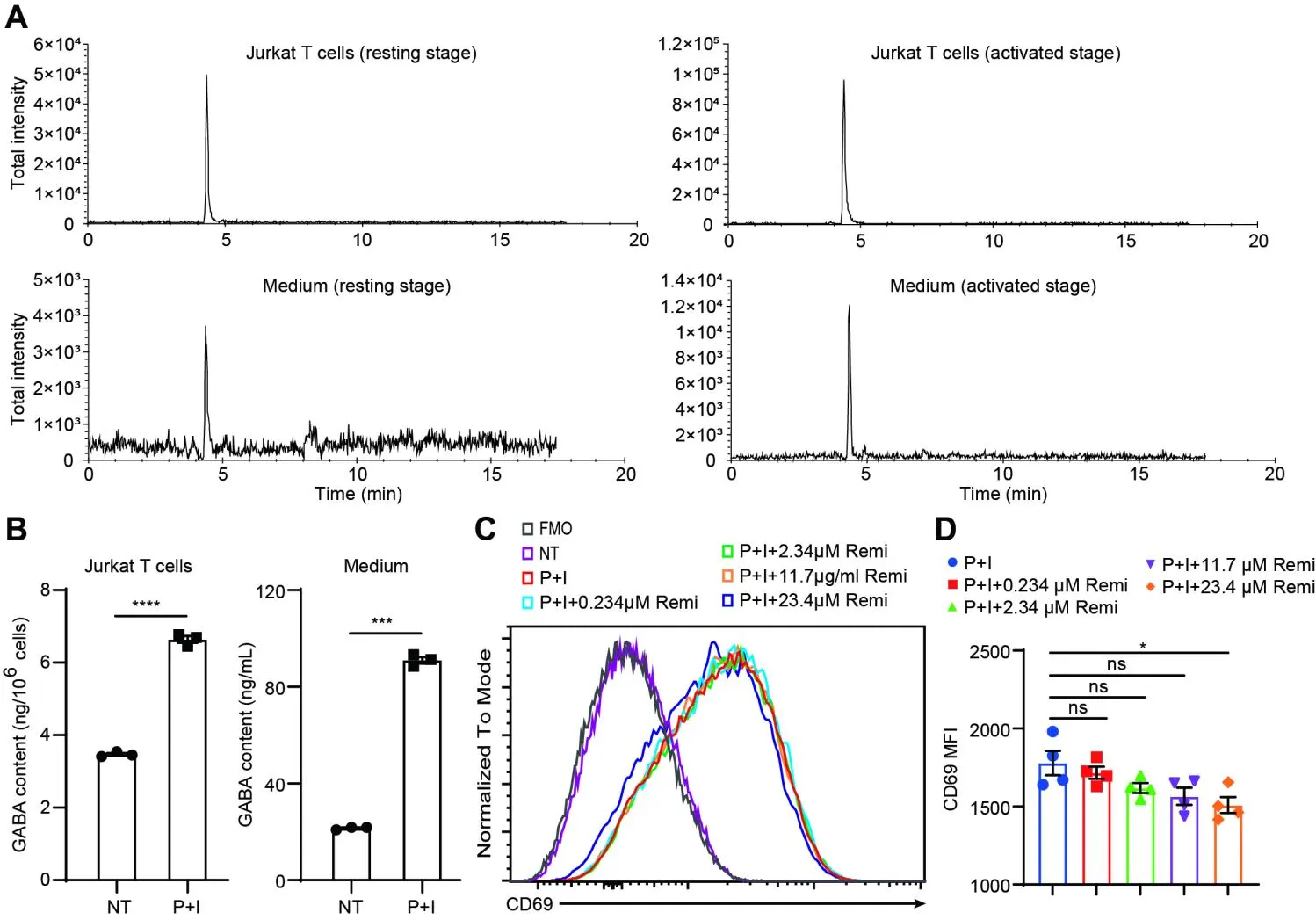
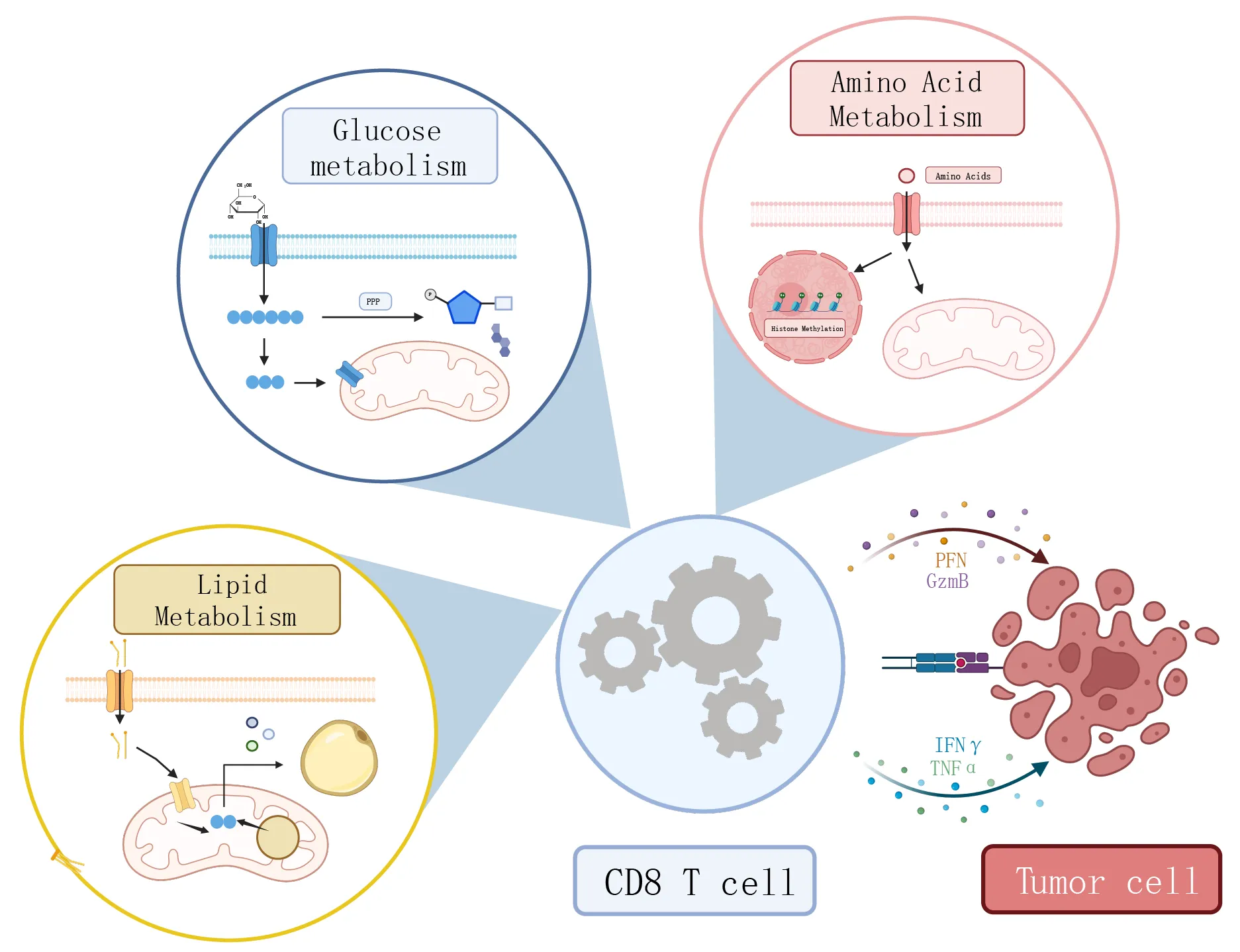
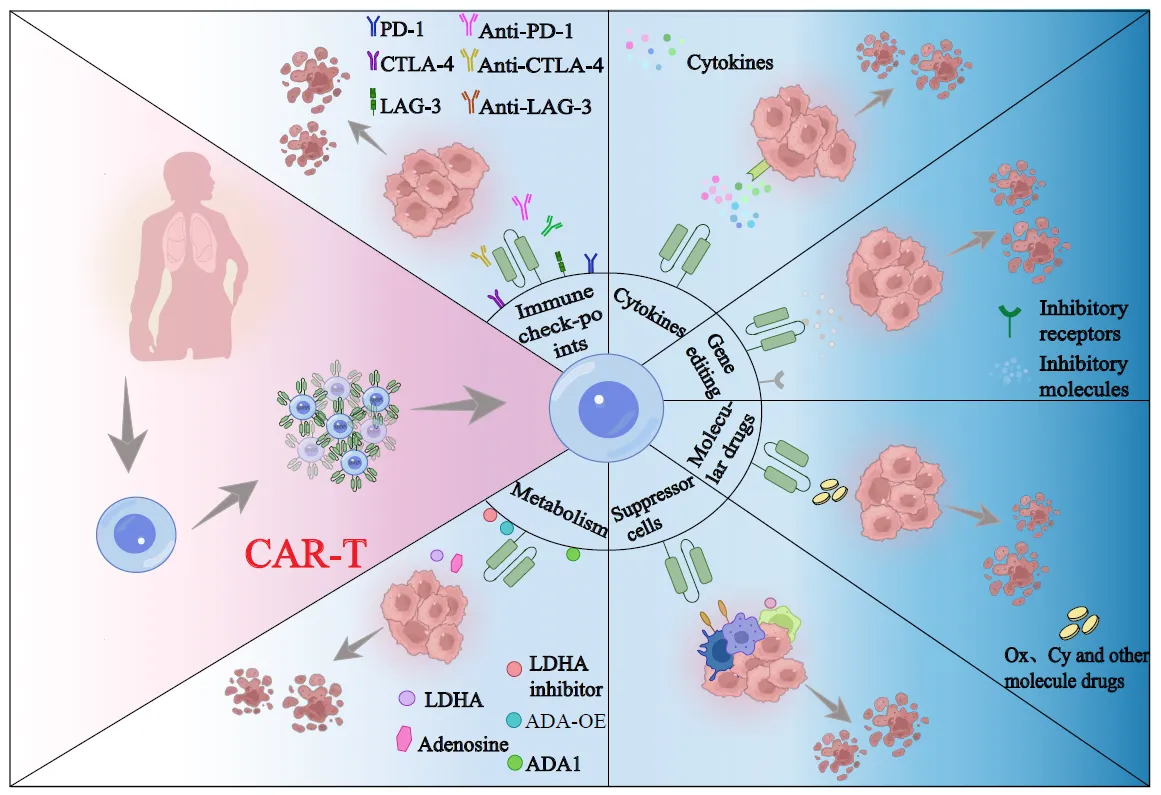
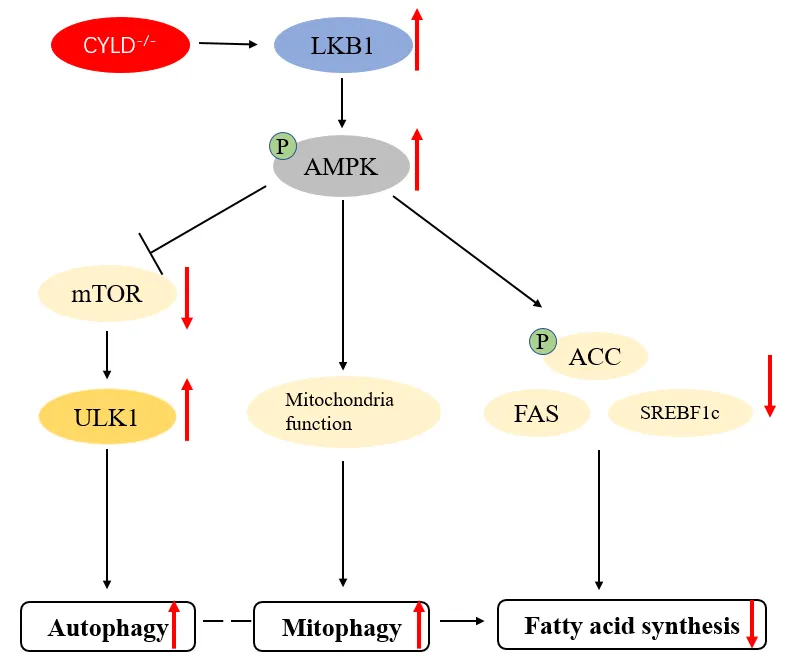
Immune Discovery
 Open Access
Open Access
ISSN: 3007-6730 (Online)
3007-6722 (Print)