Found 32 results
Open Access
Commentary
29 December 2025Breath and Life: Emerging Nanotechnologies for Cystic Fibrosis Therapy
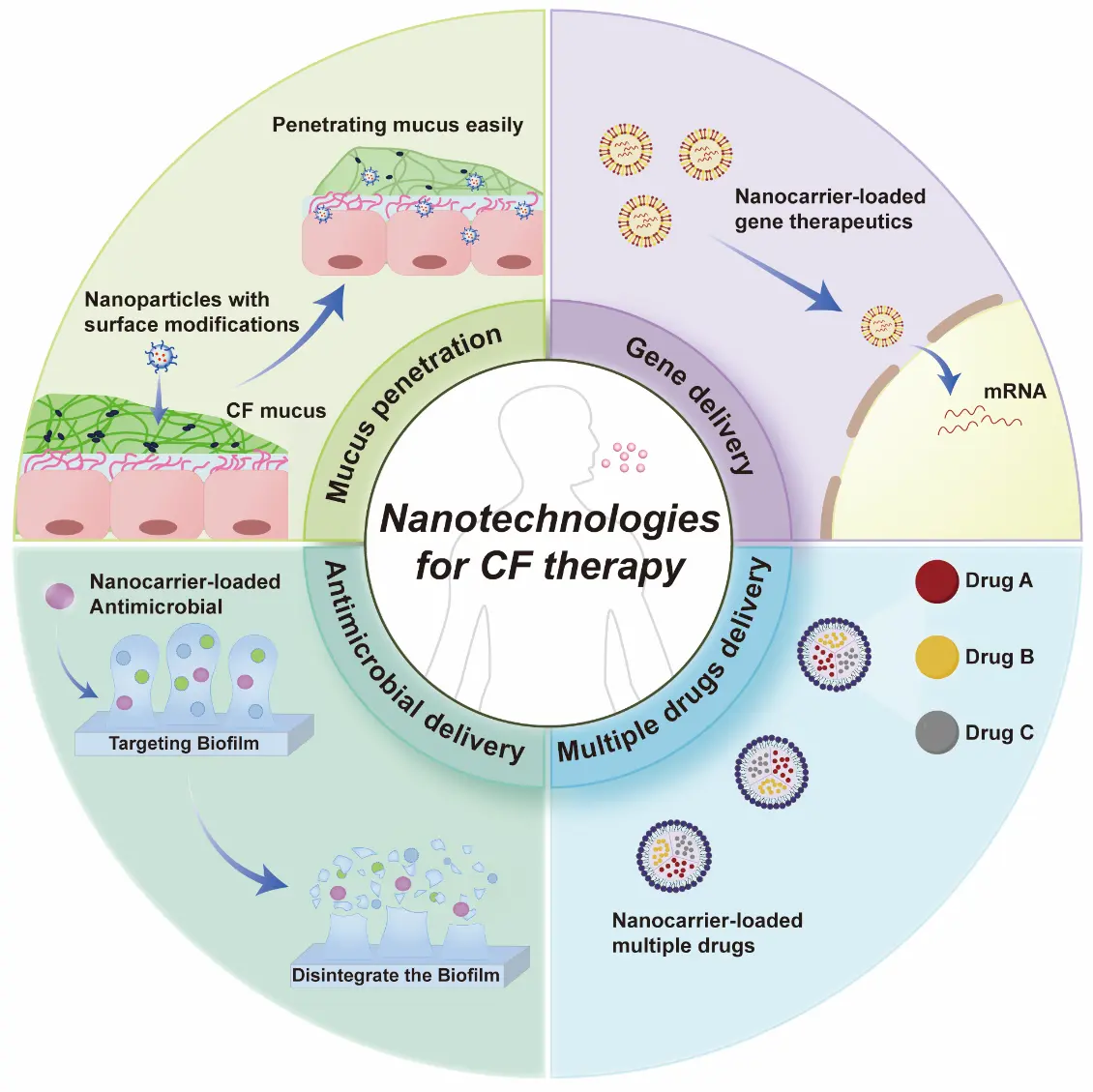
The treatment of cystic fibrosis (CF) remains challenging due to formidable biological barriers in the lungs, including thick mucus and resilient biofilms that severely limit the efficacy of conventional therapies. Nanotechnology, engineered to overcome these barriers, is emerging as a transformative approach for CF therapy. This opinion highlighted the most recent and advanced nanotechnologies, categorizing them into four strategic frontiers: (1) nanocarriers that achieve mucus penetration through surface modifications; (2) nanoplatforms for efficient delivery of genetic therapeutics; (3) nanocarriers for antimicrobial delivery to cure infections associated with CF; and (4) combinatorial nanomedicines for synchronized delivery of multiple drugs. We concluded that, with the help of these nanotechnologies, therapies for CF will now undergo a paradigm shift, moving CF from a fatal disease to a treatable and potentially curable one. Although the clinical transition is challenging, it holds immense promise for revolutionizing CF management.
Open Access
Commentary
20 October 2025Sulfatide Inhibits Growth of Fibroblasts and Is a Potential Treatment against Fibrosis
Fibrosis of vital organs such as the lungs, liver, and kidneys is a serious condition without effective causal treatment. Here, we suggest the use of the sphingolipid sulfatide and its isoform C16, which we have found to inhibit the growth of fibroblasts. In the lungs, sulfatide can be easily administered via an inhalation spray. Alternatively, fenofibrate, an anti-cholesterol drug with no major side effects, may be used, as it enhances the body’s own production of sulfatide.
Open Access
Article
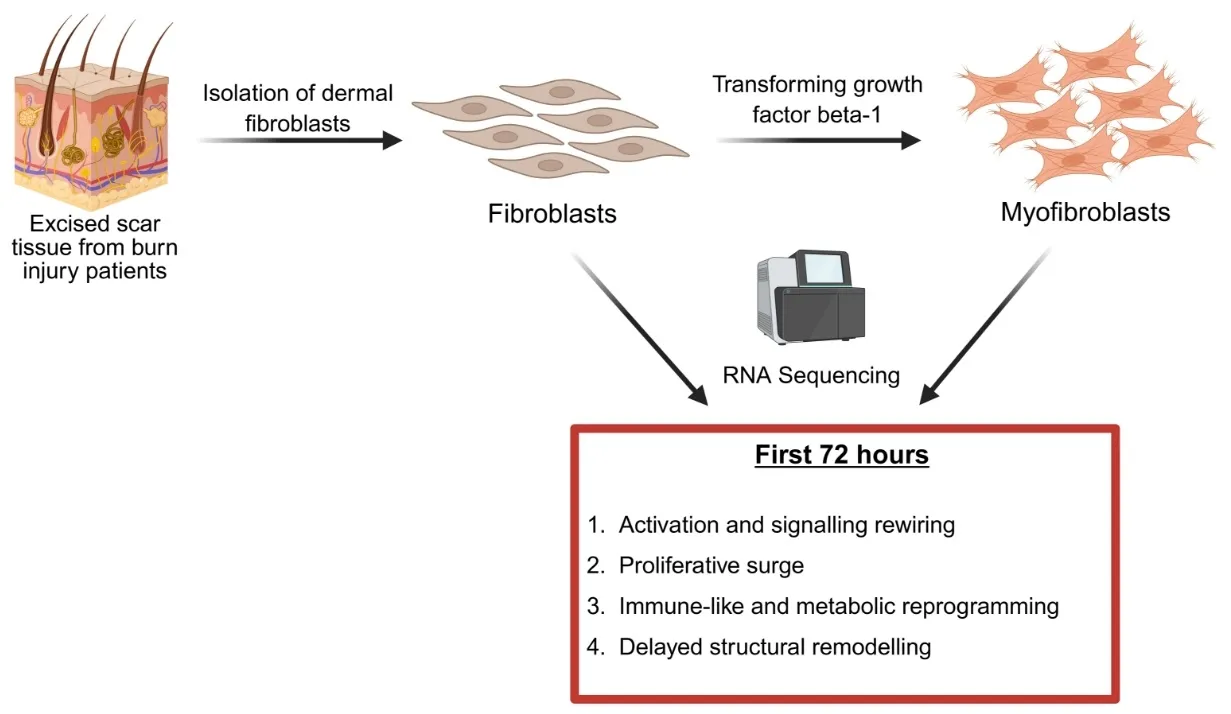
09 October 2025Identification of Pathways That Drive Myofibroblast Transformation in Hypertrophic Scars
Hypertrophic scars (HTS) are a common complication of burn injuries and are characterized by excessive dermal fibrosis driven by the transformation of resident dermal fibroblasts to profibrotic myofibroblasts. Although single cell and bulk RNA transcriptomics analysis of HTS and normal skin tissue samples were performed previously, transcriptomics of the transformation of fibroblasts to myofibroblasts has not been studied. Here, we report the data obtained from RNA sequencing of fibroblasts before and after exposure to transforming growth factor beta 1 (TGF-β1) and highlight the pathways that are up- and down-regulated during myofibroblast transformation. Our results suggest increased cellular signalling and rewiring, proliferative surge, immune-like and metabolic reprogramming, and delayed structural remodelling as four groups of events during the transformation of human primary dermal fibroblasts to myofibroblasts.
Open Access
Review
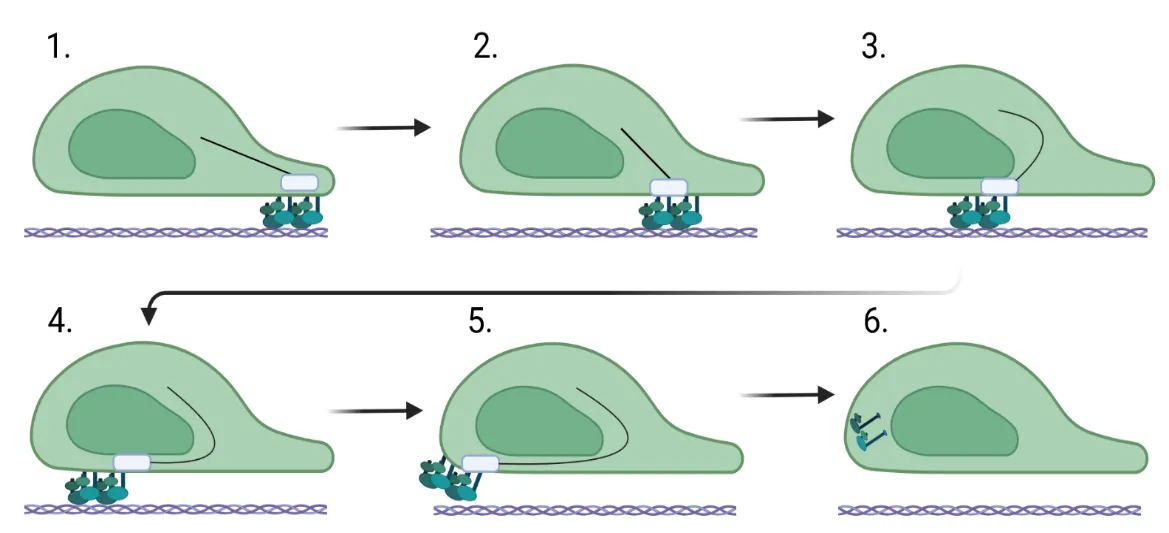
27 June 2025Fibroblast Migration in Fibrosis
Fibroblast migration is a critical factor in wound healing, but also plays a fundamental role in fibrosis. For a fibroblast to migrate, the cell must be able to assemble factors that help it crawl across the extracellular matrix. Most of this movement is facilitated through the assembly and stability of the cytoskeleton that connects focal adhesion engagement with the extracellular matrix to intracellular stress fibers that wrap around the nucleus. These intracellular stress fibers help to polarize the fibroblast and orient the nucleus in the direction it is traveling. Changes in intracellular signaling for the fibroblast to move are also required, and this is necessitated by downstream signaling mediated by sonic hedgehog, WNT/β-catenin, ROCK/Rho, and PI3K/AKT. These changes regulate the stability of the cytoskeleton and, in addition, increase the expression of genes involved in cell migration. This review assimilates what is known about the function of the cytoskeleton in migration and the role of intracellular signaling pathways in fibrosis.
Open Access
Review
27 June 2025Targeting Collagen Secretion as a Potential Therapeutic Strategy to Modulate Fibrosis
Fibrotic diseases are driven by the excessive accumulation of extracellular matrix (ECM), particularly collagens, leading to progressive tissue stiffness and organ dysfunction. While many factors contribute to fibrosis—including cytokine signaling, integrin-mediated mechanotransduction, and altered ECM degradation—the synthesis and secretion of collagen remain central bottlenecks. Collagen biosynthesis is a complex process involving extensive post-translational modification and intracellular trafficking. The export of procollagen from the endoplasmic reticulum (ER) requires Transport and Golgi Organisation 1 (TANGO1), a transmembrane organizer of ER exit sites that coordinates cargo selection, membrane remodeling, and connectivity between the ER and the ER-Golgi-Intermediate-Comaprtment (ERGIC). By assembling into ring-like structures at ER exit sites, TANGO1 builds a secretory route for bulky cargoes that bypasses conventional vesicle constraints. Loss of TANGO1 disrupts collagen secretion and causes developmental defects across various species. In fibrotic tissues, TANGO1 expression is upregulated, linking secretory machinery to pathological matrix deposition. Recent work has identified specific interfaces within the complex of TANGO1 with its vertebrate paralogue Cutaneous T-cell lymphoma-associated antigen 5 (cTAGE5) as targets for cell-permeant peptide inhibitors. Inhibitors that selectively and specifically block TANGO1 complex formation reduce collagen secretion in fibroblasts and scar formation in vivo, offering a new strategy to modulate fibrotic processes.

Open Access
Commentary
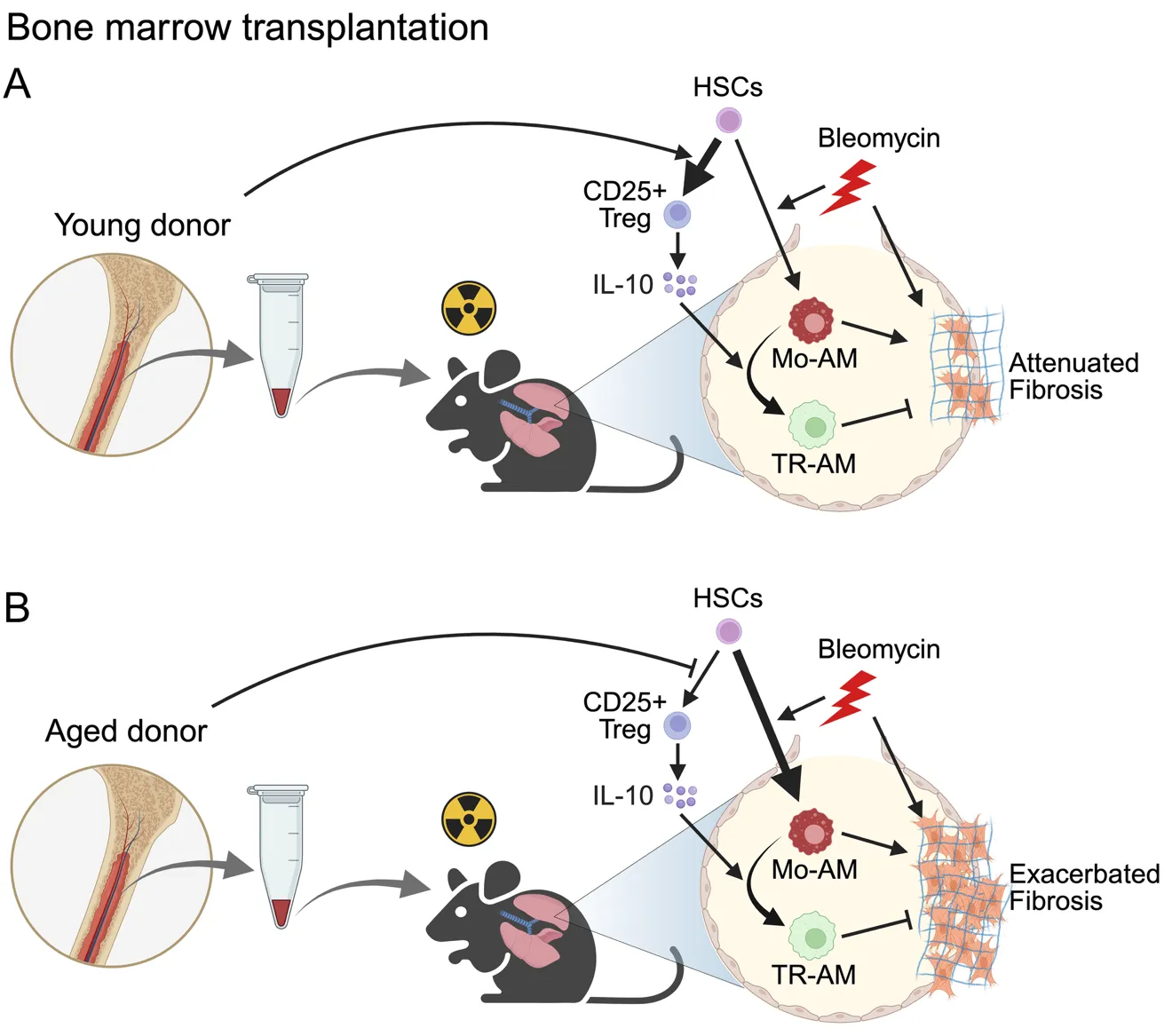
12 June 2025Bone Marrow Transplantation as a Future Therapeutic Strategy for Idiopathic Pulmonary Fibrosis: Lessons from Hematopoietic Aging
Idiopathic pulmonary fibrosis (IPF) is a fatal disease with limited therapeutic options. Lung transplantation is the only curative treatment, but it is rarely available due to a lack of suitable donors. In a recent publication in Science Immunology, Farhat et al. demonstrated that bone marrow transplantation from young donors alleviates fibrosis by restoring immune resolution in aged hosts in animal models. Aged hematopoietic cells exacerbate fibrosis through the persistence of inflammatory macrophages and impaired Treg-derived IL-10, highlighting bone marrow rejuvenation as a potential treatment strategy for IPF.
Open Access
Review
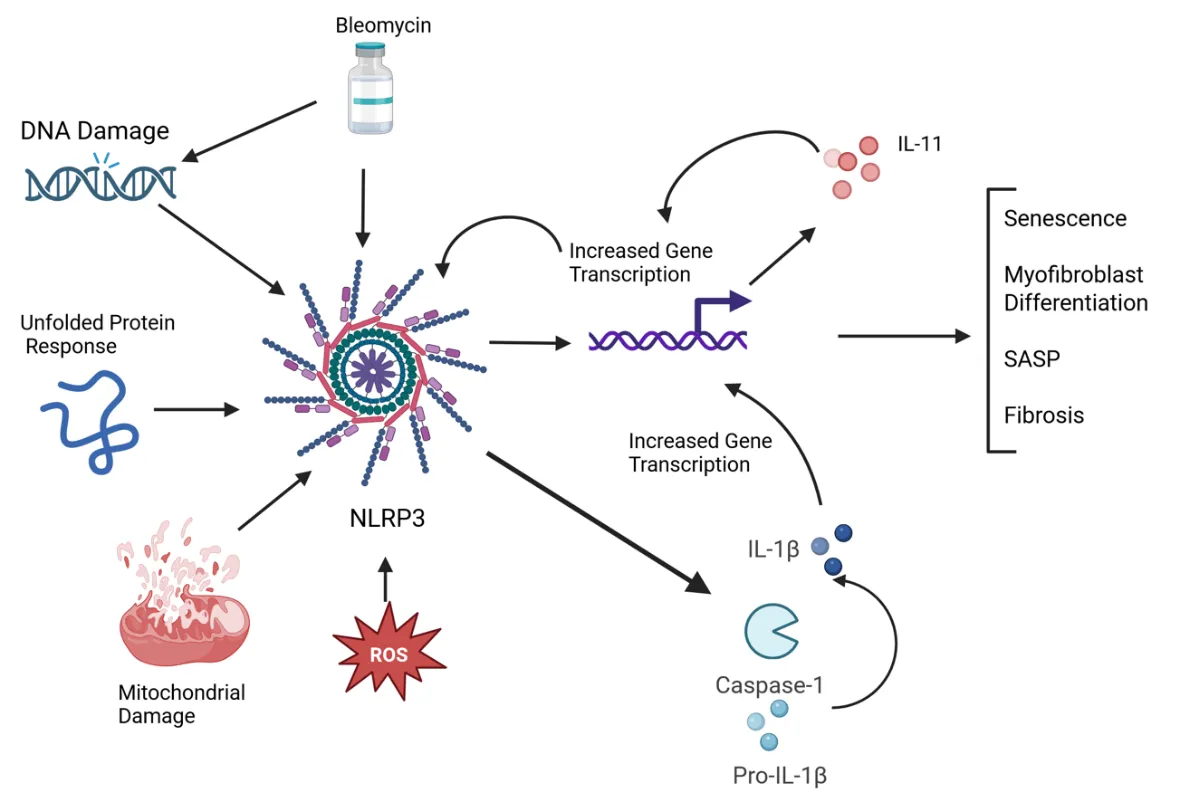
22 May 2025NLRP3 Inflammasome and IL-11 in Systemic Sclerosis Pulmonary Fibroblasts
Systemic sclerosis (SSc) is an autoimmune disease characterized by widespread fibrosis affecting multiple organ systems. There is clinical heterogeneity among patients with SSc in terms of the organs affected. However, the pathophysiology of the disease remains elusive. The NLRP3 inflammasome is upregulated in SSc and exerts its fibrotic effects through activation of caspase-1, which in turn activates a fibrotic signaling cascade, resulting in increased collagen deposition and myofibroblast transition. Recently, IL-11 has been shown to be elevated in disease and has been shown to participate in downstream signaling via the NLRP3 inflammasome. A significant number of patients with SSc will develop pulmonary involvement, termed interstitial lung disease (SSc-ILD). Though this type of pulmonary involvement is distinct from other types of pulmonary fibrosis (such as idiopathic pulmonary fibrosis), it may be a valuable model to study mechanisms of fibrosis that could apply to other fibrotic diseases. Here, we discuss recent advances in understanding the mechanisms of the NLRP3 inflammasome and IL-11 in SSc pulmonary fibroblasts. We tie together some of the recent findings, such as senescence, the unfolded protein response, and reactive oxygen species, that contribute to fibrotic pathology via modulating NLRP3 activation, possibly leading to IL-11 expression.
Open Access
Review
07 March 2025Mechanisms and Therapeutic Potential of Myofibroblast Transformation in Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible, and fatal disease with an increasing incidence and limited therapeutic options. It is characterized by the formation and deposition of excess extracellular matrix proteins resulting in the gradual replacement of normal lung architecture by fibrous tissue. The cellular and molecular mechanism of IPF has not been fully understood. A hallmark in IPF is pulmonary fibroblast to myofibroblast transformation (FMT). During excessive lung repair upon exposure to harmful stimuli, lung fibroblasts transform into myofibroblasts under stimulation of cytokines, chemokines, and vesicles from various cells. These mediators interact with lung fibroblasts, initiating multiple signaling cascades, such as TGFβ1, MAPK, Wnt/β-catenin, NF-κB, AMPK, endoplasmic reticulum stress, and autophagy, contributing to lung FMT. Furthermore, single-cell transcriptomic analysis has revealed significant heterogeneity among lung myofibroblasts, which arise from various cell types and are adapted to the altered microenvironment during pathological lung repair. This review provides an overview of recent research on the origins of lung myofibroblasts and the molecular pathways driving their formation, with a focus on the interactions between lung fibroblasts and epithelial cells, endothelial cells, and macrophages in the context of lung fibrosis. Based on these molecular insights, targeting the lung FMT could offer promising avenues for the treatment of IPF.

Open Access
Review
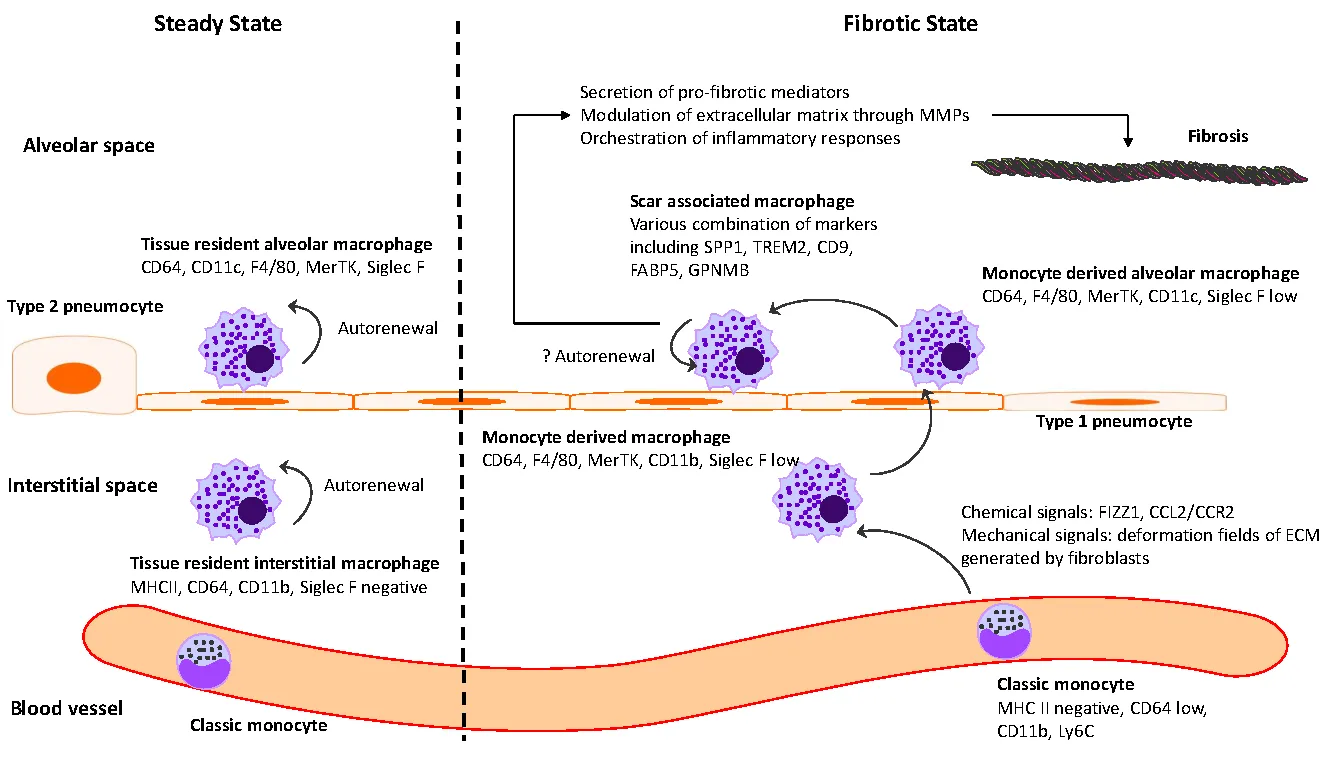
18 February 2025The Intersection between Immune System and Idiopathic Pulmonary Fibrosis—A Concise Review
Idiopathic pulmonary fibrosis (IPF) is marked by progressive alveolar destruction, impaired tissue regeneration, and relentless fibrogenesis, culminating in respiratory failure and death. A diverse array of resident and non-resident cells within the lung contribute to disease pathogenesis. Notably, immune cells, both resident and recruited, respond to cues from sites of lung injury by undergoing phenotypic transitions and producing a wide range of mediators that influence, initiate, or dictate the function, or dysfunction, of key effector cells in IPF pathology, such as alveolar epithelial cells, lung fibroblasts, and capillary endothelial cells. The role of the immune system in IPF has undergone an interesting evolution, oscillating from initial enthusiasm to skepticism, and now to a renewed focus. This shift reflects both the past failures of immune-targeting therapies for IPF and the unprecedented insights into immune cell heterogeneity provided by emerging technologies. In this article, we review the historical evolution of perspectives on the immune system’s role in IPF pathogenesis and examine the lessons learned from previous therapeutic failures targeting immune responses. We discuss the major immune cell types implicated in IPF progression, highlighting their phenotypic transitions and mechanisms of action. Finally, we identify key knowledge gaps and propose future directions for research on the immune system in IPF.
Open Access
Review
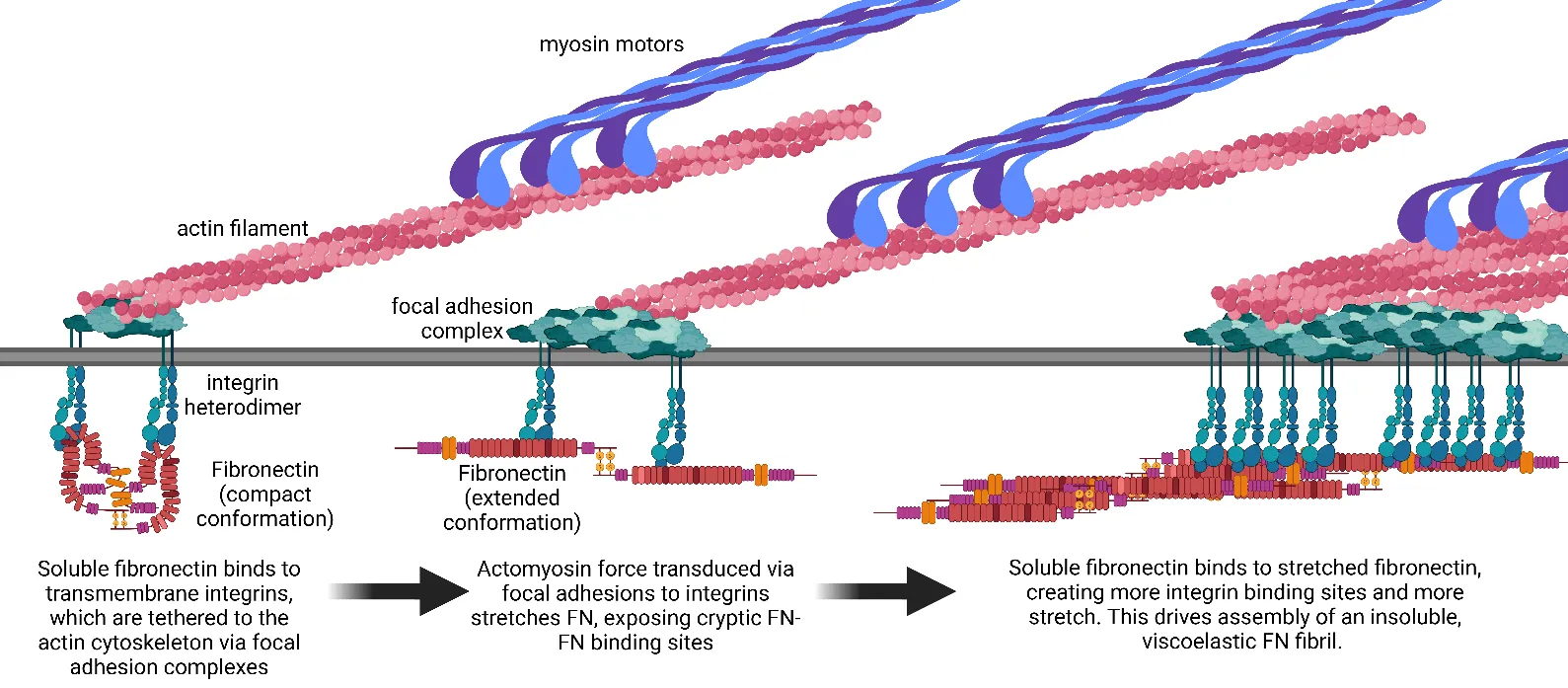
08 February 2025Mechanics and Synergistic Signaling of Fibronectin, Integrins, and TGF-β Isoforms
Fibrotic diseases such as pulmonary fibrosis, hepatic fibrosis, chronic kidney disease, and cancer are marked by an excess accumulation of extracellular matrix (ECM). This process involves the assembly of the ECM protein fibronectin (FN) into insoluble fibrils. FN fibril assembly is highly linked with integrin signaling, TGF-β1 signaling, and cellular contractility. This linkage consists of four stages: (i) Integrin binding and contractile forces facilitate the assembly of FN into insoluble fibrils; (ii) assembled FN fibrils bind the large latent complex of TGF-β1; (iii) activation of TGF-β1 from the latent complex requires integrin binding and contractile forces; and (iv) active TGF-β1 increases contractility, integrin expression, and FN assembly. The significance of integrin signaling and TGF-β1 signaling in fibrotic diseases is well-appreciated, as numerous clinical trials targeting integrins or TGF-β1 have been reported. However, despite a clear effort to target integrins and TGF-β1 clinically, the vast majority of these trials have failed or have been terminated. These suggest a potentially incomplete understanding of the synergistic effects of these pathways. Here we present a review of both FN fibrillogenesis and TGF-β1 signaling, as well as current opinions of under-explored areas of crosstalk related to these pathways that may explain why these have not been successfully targeted in many disease states including fibrosis.