Issue 2, Volume 2 – 6 articles
Cover Story (View full-size image):
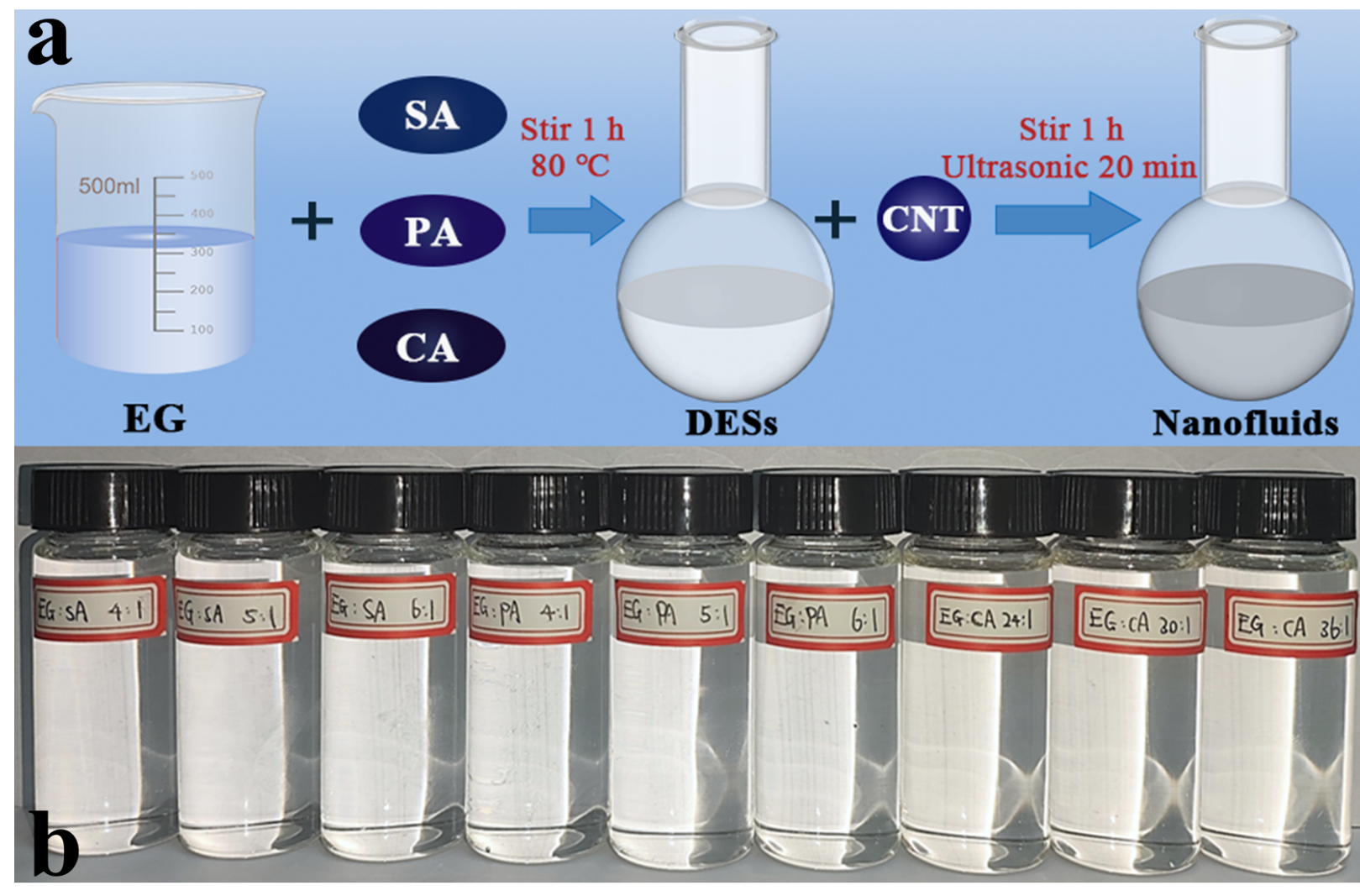
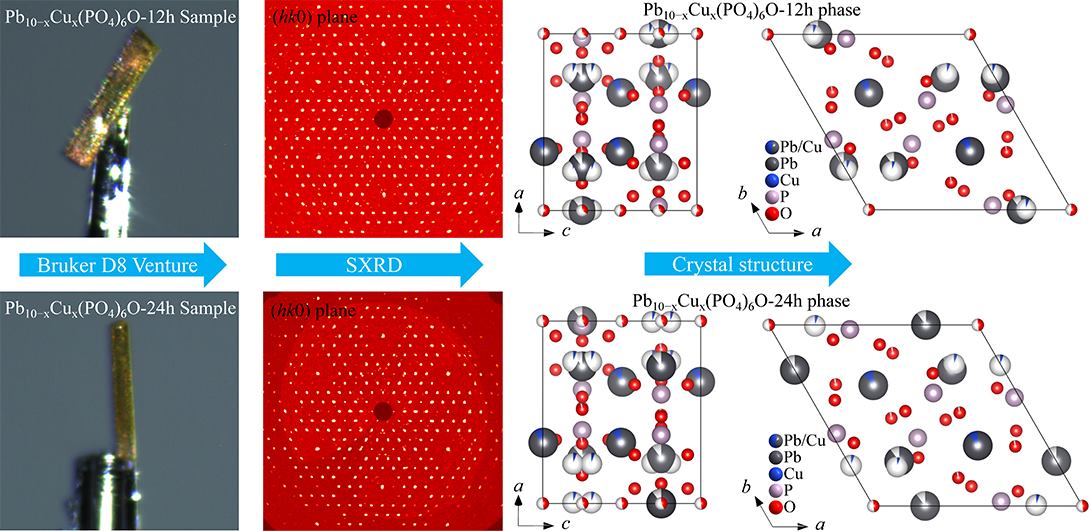
It was found that the four natural apatite samples comprised one type of fluorapatite and three types of hydroxyapatite, all crystallized in the hexagonal system, P63/m space group. The refined crystal has been compared with prevailing structure models during the last 20 years. The crystal structure of our successfully synthesized copper-doped lead apatite contains three types of disordered atoms: O vacancy atoms, Pb/Cu split atoms and Pb/Cu co-occupying atoms. The topological analysis of the present structure model indicates that the it can be characterized by three single-shell clusters: P1 (1) (1@4), Pb1/Cu1 (1) (1@9), and Pb2 (1) (1@7).