1. Introduction
Opuntia ficus-indica is a native plant from Mexico, characterized by its oval, flattened cladodes—modified stems that perform photosynthesis and are commonly mistaken for leaves [
1]. In Mexico, twenty-five states, obtain an annual production of 837,267 tons of cladodes [
2], of which 70% is sold (fresh or processed) to the food and pharmaceutical industry as brines, juices, jellies, among others [
3]. The remaining 30% represents a critical excess [
4] which is typically discarded by producers, leading to a large quantity of daily generated waste. Due to its abundance, low cost, and renewable nature, cladode biomass represents a promising alternative substrate for microbial cultivation, enzymatic processes, and other biotechnological applications [
5,
6]. Mexican producers have recognized the importance of developing strategies to utilize this surplus, not only to reduce waste but also to generate added value from underused resources [
7,
8].
Cladodes contain complex polysaccharides such as cellulose (21.6%), hemicellulose (21%), lignin (3.6%) [
9] and mucilage (33%) [
10]. Cellulose and hemicellulose represent an energy-rich carbon source due to glucose molecules (C
6H
12O
6) bound by β-glucosidic bonds. The mucilage is a heteropolysaccharide with high water activity (>0.8) [
11] that contains simple sugars (rhamnose, xylose, arabinose, glucose and galactose) bound to galacturonic acid by β-glucosidic bonds [
10,
12], all values expressed on a dry matter basis. The presence of this glycosidics bonds makes cladode flour (CF) susceptible to the hydrolysis process. Moreover, there is an interest in using purified and/or crude enzymes as a good hydrolyzing agent for avoiding the use of corrosive acids in this critical stage, considered as a hydrocolloid with industrial potential. The mucilage is a non-gelling agent with elastic properties similar to those of polymers such as xanthan gum and carboxymethylcellulose (CMC), with a high ability to increase the viscosity of aqueous systems [
7,
13,
14]. There are reports of extraction methodologies, chemical composition, functional, physicochemical and rheological properties of mucilage [
7,
8,
10,
14,
15,
16,
17,
18]. However, cladodes have a low total solids content (~4–7% on fresh weight), which may be a limitation in fermentation processes that require high biomass input. Additionally, lignin content increases with the age of the cladode, which is why young cladodes are preferred for consumption and processing [
10]. Additionally, despite the extensive characterization of mucilage, there are no reports of changes in hydrolytic activity and flow behavior due to the growth of wild hydrolytic bacteria.
The aim of this study was to evaluate the fermentation dynamics of two wild hydrolytic microorganisms and their effect on the flow behavior of a culture medium formulated with
Opuntia ficus-indica cladode flour.
2. Materials and Methods
2.1. Vegetal Material
Opuntia ficus-indica Atlixco variety cladodes of 12 months of age were collected in an experimental field. The fresh cladodes were cut into pieces (1 cm
2) using an industrial vegetable cutter (3/8 inch blade, Hobart, FP-350, Troy, OH, USA) and dried at 80 °C/24 h in a steam trays dryer (Jersa, FT-DCV-02, Guadalajara, Mexico). Subsequently, dry cladodes were ground (Pulvex, 200, Naucalpan, Mexico) to obtain a flour with a particle size of 1500 µm, and stored in sealed plastic bags (25 °C for 1 week) for culture medium preparation and further analysis.
2.2. Cladode Medium Preparation
A suspension (250 mL) was prepared by adding cladodes flour (20%) to mineral solution (MS). The MS was prepared by the addition of distilled water (225 mL) to a mixture of mineral salts (50:50, 25 mL) consisting of mineral solution I (0.6% K
2HPO
4) and mineral solution II (1.2% NaCl, 1.2% (NH
4)
2SO
4, 0.6% KH
2PO
4, 0.12% CaCl
2 and 0.25% MgSO
4·7H
2O) [
19]. All ingredients were homogenized mechanically and adjusted to pH 6.5 using a potentiometer (Oakton, pH 11 series, Singapore, Singapore). The mixture was sterilized at 121 °C/15 min.
2.3. Proximate Composition Analysis of Cladode Flour
Ash, titratable acidity, moisture and pH of cladode flour were analyzed according to AOAC 923.03, 942.15, 984.25 and 981.12, respectively [
20,
21]. Total carbohydrates [
22], free reducing sugars [
23], and minerals (Ca, K, Na, P, Mg) [
24] were analyzed. Swelling capacity (SC) and water holding capacity (WHC) were determined following the method of [
25], with minor modifications. Briefly, 1 g of cladode flour was mixed with 10 mL of distilled water in a graduated cylinder and allowed to stand at room temperature for 24 h. SC was expressed as the volume increase (mL water/g DM). For WHC, the hydrated sample was centrifuged at 3000 rpm for 20 min, and the retained water was measured gravimetrically (g water/g DM). For color measurements, a colorimeter (HunterLab, 4500L, Reston, VA, USA) was used with 13 g of sample considering L
∗, a
∗ and b
∗ values.
2.4. Isolation and Molecular Identification of Wild Hydrolytic Bacteria
2.4.1. Isolation
A homogenous slurry was set of decayed cladodes; an aliquot was then prepared to seed microorganisms using solid medium added with 1% CMC as a selective medium at 37 °C/24 h. The first visualized colonies were separated and re-seeded in solid media added with CMC and CFM (1%, pH 6.5).
2.4.2. Morphological Characterization and Qualitative Hydrolytic Activity Determination
The shape, size, color and texture of the obtained colonies were visualized using a stereoscope (10×, Leica, EZ4D, Singapore). The shape of the cells was determined using a microscope (100×, Olympus, CX31RBSFA, Tokyo, Japan). Congo tests were performed to ensure hydrolytic activity by observation of halos degradation according to the Teather and Wood protocol [
26]. Gram tests were performed according to Bartholomew and Finkelstein (1958) [
27] protocol.
2.4.3. Molecular Identification
Molecular identification of hydrolytic microorganisms was performed by analysis of the 16S ribosomal gene sequence (Thermo Fisher, Waltham, MA, USA). The genomic DNA used as a template was obtained from 5 mL of a cell culture growth in nutrient broth (Sigma, St. Lois, MO, USA) at 37 °C/24 h. For genomic DNA extraction, a CTAB 2% buffer was used [
28]; subsequently, a purification with the Gen EluteTM PCR Clean-Up kit protocol (Sigma) was performed. The obtained fragments were amplified using oligonucleotides 16S (5′AGA.GTT.TGA.TCC.TGG.CTC3′) and 16SR (5′CGG.GAA.CGT.ATT.CAC.CG3′). The amplified fragments were cloned into pGEM-T vector system I (Promega, Madison, WI, USA) for recombinant plasmids construction. Transformation with JM109 competent cells (Promega) was completed, and then, a purification of plasmidic DNA with GeneluteTM Plasmid Miniprep kit protocol. The pure plasmidic DNA was quantified using a Nanodrop (2000, Waltham, MA, USA), adjusted to 150 ng/µL and sent to sequencing. The sequencing was carried out with Macrogen Inc. (Seoul, Republic of Korea). The results of the sequences (in Fasta format) were edited excluding the vector using Geneious 5.4.8 software [
29] and the resulting sequences were used to perform a BLASTn from NBCI [
30] and identified with the generic and/or specific name according to the percentage of similarity to the reported in a database.
2.5. Growth of Wild Hydrolytic Bacteria in Cladode Flour Medium
Two bacteria were grown at 37 °C/6 h with agitation at 200 rpm starting from 1 mL of glycerol in 9 mL of 1% CFM as the first pass. A second growth pass was performed with the resulting 10 mL mixed with 50 mL of 20% CFM for 12 h. Finally, an aliquot was taken for the third pass, starting with a cell population of 20 × 10
6 cell/mL (250 mL of medium). The growth was performed for 168 h, sampling every 24 h during growth.
2.6. Protein Determination in Cladode Flour Fermentation Using Bradford Assay
Soluble protein concentration was determined throughout the fermentation process using the Bradford method [
31], adapted for samples derived from cladode flour fermentation. At each sampling point (0, 6, 12, 24, 48, 72, 120, and 168 h), 5 mL of culture broth was collected and centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was recovered and used for protein quantification.
Briefly, 100 μL of sample supernatant was mixed with 1 mL of Bradford reagent (Coomassie Brilliant Blue G-250, 0.01%
w/
v in 4.7% ethanol and 8.5% phosphoric acid) in a test tube. After 5 min of incubation at room temperature, the absorbance was measured at 595 nm using a UV-Vis spectrophotometer (JENWAY
® model 6715, Jenway, Cambridge, UK). A standard curve was constructed using bovine serum albumin (BSA) solutions ranging from 0 to 25 mg/mL, and the soluble protein concentration in the samples was expressed in mg/mL.
2.7. Specific Hydrolytic Activity Determination
The specific extracellular hydrolytic activity of wild bacteria was determined by correlating free reducing sugars and soluble protein, and expressed in International Units according to Equation (1):
where, International Units = amount of enzyme capable of releasing 1 µmol of sugar per minute under the condition tested (IU), Sugar equivalent = free reducing sugars determined (µmol), Protein = soluble protein determined (mg), Time = reaction time (min).
The enzyme preparation used for viscosity reduction was a crude extracellular enzyme extract, obtained directly from the culture supernatant of wild bacteria grown in CFM, without any purification steps. Hereafter, this preparation is referred to as “crude enzyme extract” throughout the text.
It is important to note that the protein values used to calculate specific activity correspond to the total extracellular protein content in the supernatant, including both enzymatic and non-enzymatic fractions. As bacterial growth increases, total protein rises due to the secretion of non-specific proteins, which may dilute the calculated IU/mg values. Therefore, specific activity is interpreted as a relative indicator of hydrolytic efficiency rather than an absolute measure of enzyme concentration.
2.8. Rheological Analysis
The viscosity of the cladode flour medium (CFM) obtained during the growth of wild bacteria was determined using a digital viscometer (SC4-16 needle, RVDV-III U; Brokfield Engineering Laboratory Inc., Middleboro, MA, USA) at 25 °C. The behavior was determined by recording the shear stress values (τ, Pa) needed to deform the samples with increasing (0.003 to 66.7 s
−1) shear rate values (γ, s
−1) and in descending order to obtain both the upward and downward curves, respectively. Data from both curves were adjusted to the Herschel-Bulkley equation (
Equation (2)) characterizing the flow behavior of CFM degraded by wild bacteria.
where:
τ
0 = yield stress (Pa)
n = flow behavior index (dimensionless)
K = consistency coefficient (Pa)
The parameters n and K were obtained from the log (τ − τ
0) versus log (g) diagram. The yield stress was calculated by Casson equation (τ
0.5 = τ
00.5 + ηγ
0.5).
2.9. Experimental Design
A factorial design 4 × 2 was established, evaluating the following factors (
). The response variables evaluated were yield stress (τ
0), consistency coefficient (K), flow index (n), apparent viscosity (η), bacterial growth (cel/mL), total, specific hydrolytic activity (IU), and free release sugars (g/L).
.
Factorial design 4 × 2 of the effect of fermentation time and type of microorganism on the physicochemical characteristics of a medium formulated with cladode flour.
| Exp |
Factors |
Response Variables |
| Time (h) |
Strain |
τ0 (Pa) |
K (cP) |
n |
η (Pa·s) |
Bg |
SHA |
FRS |
| 1 |
0 |
A. pitti |
δ1 |
β1 |
α1 |
Σ1 |
O1 |
R1 |
V1 |
| 2 |
24 |
A. pitti |
δ2 |
β2 |
α2 |
Σ2 |
O2 |
R2 |
V2 |
| 3 |
96 |
A. pitti |
δ3 |
β3 |
α3 |
Σ3 |
O3 |
R3 |
V3 |
| 4 |
168 |
A. pitti |
δ4 |
β4 |
α4 |
Σ4 |
O4 |
R4 |
V4 |
| 5 |
0 |
B. subtilis |
δ5 |
β5 |
α5 |
Σ5 |
O5 |
R5 |
V5 |
| 6 |
24 |
B. subtilis |
δ6 |
β6 |
α6 |
Σ6 |
O6 |
R6 |
V6 |
| 7 |
96 |
B. subtilis |
δ7 |
β7 |
α7 |
Σ7 |
O7 |
R7 |
V7 |
| 8 |
168 |
B. subtilis |
δ8 |
β8 |
α8 |
Σ8 |
O8 |
R8 |
V8 |
2.10. Statistical Analysis
The effect of the two parameters tested, time (0 h, 24 h, 96 h, 168 h) of fermentation, strain (
A. pitti,
B. subtilis), and their interaction, was statistically analyzed in terms of the rheological behavior. All response variables were examined with statistical software (Minitab v. 15, Inc., State College, PA, USA) with a 5% test of significance.
3. Results
3.1. Characterization of Cladode Flour
The physicochemical composition of cladode flour (CF) is presented in
.
Ashes were similar (24.04%) to those reported for other authors [
7,
12] for cladode flours from other varieties (16–20%), probably because the cladodes of this work were part of a mineralized experimental lot where fertilization source was an inorganic fertilizer that provided nitrogen, phosphorus and potassium, based on urea, triple superphosphate and potassium chloride [
32]. CF presented a higher content of Ca
2+, Mg
2+ and K
+ compared with other reports [
12,
17,
33,
34,
35] while the concentration of Na
1+ and P
3+ was slightly. Several researches have shown the importance of calcium and its compounds (calcium oxalate and calcium carbonate) present in the cell walls of
Opuntia ficus-indica cladodes, because they are fundamental compounds that form the mucilage network in young cladodes (less than a year) [
36].
.
Proximate composition and physical characteristics of cladodes flour (dry matter) from Opuntia ficus-indica var. Atlixco (12 months) a.
| Parameters |
Value |
| Ash (g/100 g) |
24.04 ± 0.34 |
| Moisture (g/100 g) |
6.13 ± 0.49 |
| pH |
5.06 ± 0.01 |
| Titratable acidity (g/100 g) |
0.22 ± 0.07 |
| Total carbohydrates (g/100 g) |
18.15 ± 0.10 |
| Free reducing sugars (g/100 g) |
4.15 ± 0.00 |
| Swelling (mL water/g DM b) |
10.00 ± 3.00 |
| Water holding capacity (g water/g DM b) |
6.80 ± 0.40 |
Color
(dimensionless)
|
L* |
68.09 ± 0.35 |
| a* |
−1.82 ± 0.04 |
| b* |
23.99 ± 0.22 |
Minerals
(g/100 g)
|
Ca |
4.78 ± 0.01 |
| Mg |
0.98 ± 0.01 |
| K |
3.36 ± 0.01 |
| Na |
0.006 ± 0.001 |
| P |
0.064 ± 0.01 |
A moisture of 6.13% was detected, this value is desirable due to microbial growth and hygroscopicity is prevented (lower than 10%), improving the shelf life of the flour. Atlixco variety exhibited a high pH value (>5) compared with other varieties (
i.e.,
Opuntia ficus-indica amylocea and
Opuntia ficus-indica inermis, pH ~ 3), explained by the age of cladodes and the concentration of many organic acids, such as malic, citric and oxalic acids [
25,
33]. Related to hydration properties, the CF swelling power obtained (10.00 ± 3 mL/g) was higher, while the value of water retention capacity (WRC) was similar (6.80 ± 0.40 g/g) compared with the data obtained by Ayadi et al. (2009) [
25] (7.36 ± 0.07 mL/g and 6.85 ± 0.03 g/g, respectively). The CF presented a better ability to bind to water molecules. Color parameters showed flour with high luminosity (L
∗ = 68.09 ± 0.35) and a yellow-green color (b
∗ = 23.99 ± 0.22, a
∗ = −1.82 ± 0.04). The color parameters were similar to those reported by Sáenz (1997; 2010) [
12,
37] (L
∗ = 73.37, b
∗ = 26.1 and a
∗ = −5.20) and Ayadi et al. (2009) [
25] (L
∗ = 67.49, b
∗ = 25.15 and a
∗ = −8.17).
In addition, although the values of total carbohydrates and free reducing sugars represent a fraction of the flour (18.15 ± 0.10 g·100 g⁻¹ and 4.15 ± 0.00 g·100 g⁻¹, respectively), the remaining percentage up to 100% dry basis is mainly attributed to structural carbohydrates such as cellulose, hemicellulose, lignin, and pectins. This estimation is a common practice in biomass studies [
7,
38]. In fact, several authors have reported that
Opuntia ficus-indica cladodes contain approximately 9–14% cellulose, 20–25% hemicellulose, and 6–14% lignin, in addition to mucilage and pectins as key components of their cell-wall architecture [
39,
40]. These reports support structural carbohydrate content in
Opuntia ficus-indica cladodes flour (~60%).
3.2. Identification of Wild Hydrolytic Bacteria
3.2.1. Isolation
Producing hydrolytic enzyme strains (four) were obtained from decayed cladodes. All strains grew well at 37 °C on carboxymethylcellulose (CMC) and cladode flour medium (CFM) under aerobic conditions during the first 24 h. Microbial hydrolytic enzymes production is influenced by factors including inducer substrate, type of strain used and reaction conditions (temperature, pH,
etc.).
3.2.2. Morphological Characterization and Qualitative Determination of Hydrolytic Activity
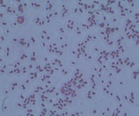
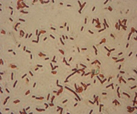
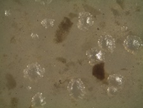
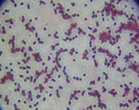
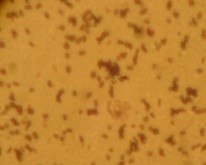
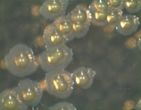
The microorganisms were identified as bacteria by microscopic observation (
) and presented two morphologies: cocci and bacilli. An identification code was assigned to each of them: B400, B401, B402 and B403. The colonies presented circular forms (B400 and B402) and irregular forms (B401 and B403) with white, orange and yellow coloration. All bacteria had Gram (–) staining and only B400 and B401 presented hydrolytic activity, as confirmed by degradation halos in solid medium with 1% of CF. Two hydrolytic bacteria were chosen for molecular identification; specific hydrolytic activity quantification was made during growth, as well as the rheology in cladode flour medium (CFM).
3.2.3. Molecular Identification
When performing the identity search for each sequence obtained, the bacterium B400 presented 100% identity with
Acinetobacter pittii, whereas the bacterium B401 presented 99% identity with
Bacillus subtilis. Some studies have reported the identification of new bacteria identified as
Pseudomonas,
Staphylococcus,
Acinetobacter and
Bacillus with cellulolytic activity isolated from gut termite [
41], sawdust [
42] and from
Opuntia ficus-indica [
43]. The isolated bacteria from this work correspond to the genera already reported to have hydrolytic activities, specifically cellulolytic.
3.3. Growth and Specific Hydrolytic Activity Determination of Wild Bacteria
Acinetobacter pittii and
Bacillus subtilis showed satisfactory growth in CFM, although they exhibited an adaptation phase of at least 24 and 48 h, respectively (
).
. Growth curves for <em>A. pittii </em>(black square) and <em>B. subtilis </em>(gray square). Specific hydrolytic activity (IU) for <em>A. pittii </em>(black bar) and <em>B. subtilis </em>(grey bar). Free reducing sugars for <em>A. pittii </em>(black diamond) and <em>B. subtilis </em>(grey diamond). Average of two replicated. Specific activity (IU·mg<sup>−1</sup>) was calculated based on total extracellular protein. The observed decrease reflects enzyme dilution due to non-enzymatic protein accumulation, not a loss of hydrolytic capacity.
A two-phase trend in free reducing sugars was observed. In the first 24–48 h, an increase in reducing sugar concentration—particularly in
A. pittii—coincided with the peak of extracellular hydrolytic activity, reaching values of 0.22 IU·mg⁻¹ for
B. subtilis and 0.21 IU·mg⁻¹ for
A. pittii. While our study did not include identification of the specific monosaccharides released or direct enzyme characterization, the hydrolytic behavior observed is consistent with the general degradation of polysaccharides in the matrix. Previous studies have reported the ability of
Bacillus subtilis and
Acinetobacter species to produce extracellular hydrolytic enzymes such as cellulases, pectinases, and xylanases under similar conditions [
44,
45,
46]. Additionally,
Opuntia ficus-indica cladodes have been shown to respond effectively to enzymatic mixtures containing cellulases and xylanases, facilitating pectin extraction and matrix disruption [
47]. After this time, the consumption of all released sugars and the decrease of hydrolytic activity were observed, coinciding with the increase in bacterial growth.
Acinetobacter pittii exhibited exponential growth, reaching a maximum of 4.68 ± 0.66 × 10
9 cell/mL after 144 h, while
Bacillus subtilis reached a final population of 3.92 ± 0.20 × 10
9 cell/mL.
3.4. Rheological Analysis
All samples showed pseudoplastic behavior, a characteristic of compounds with high molecular weights such as mucilage (3.3 × 10
4 kDa). In general, CFM with
A. pittii and
B. subtilis growth (
a and
b, respectively) showed shear thinning behavior due to deformation. The viscosity curves decreased with an increasing shear rate (0.003–66.7 s
−1). The slight decrease in viscosity was reached when γ ≥ 29 s
−1 (
).
. Steady shear viscosity measurement of cladode flour medium at different times (0 h, solid circle), (6 h, solid square), (12 h, solid diamond), (24 h, solid triangle), (48 h, empty circle), (96h, empty square), (120 h, empty diamond), (144 h, empty triangle), (168 h, solid cross) for (<strong>a</strong>) <em>A. pittii </em>and (<strong>b</strong>) <em>B. subtilis </em>growth. Average of two replicates.
In addition, the diagrams showed a progressive decrease in the viscosity of the medium as a function of the growth time of wild bacteria, related to the release of hydrolytic enzymes that degrade the network of the raw polymeric material. When bacteria begin to grow using monosaccharides available in the medium, they excrete potent enzymes (exo-glucanases, endo-glucanases and β-glucosidades) that break down complex polysaccharides, causing a decrease in viscosity (
) and releasing more monosaccharides into the medium, which are consumed by microorganisms to grow exponentially. In spite the increase in the concentration of soluble protein (0.20–2.28 and 0.27–1.50 mg/mL for
A. pittii and
B. subtilis, respectively) as an indicative of the production of enzymes, the activity decreased because the specific hydrolytic activity is subjected to the released amount of sugars into the medium, which were consumed by the bacteria over time.
After 12 h, an increase in the viscosity was observed for medium, most notably for
B. subtilis, which was not observed for
A. pitti (
), presumably due to the change of pH of the medium or to the presence of divalent ions like calcium.
. Apparent viscosity (γ = 29 s<sup>−1</sup>) for <em>A. pittii </em>(black bar) and <em>B. subtilis </em>(grey bar). Average of two replicate.
The viscosity of CFM obtained at 6 h of growth for
A. pittii and
B. subtilis was 1.6 ± 0.05 and 1.4 ± 0.01 Pa·s, respectively. The progressive decrease in viscosity during bacterial growth strongly suggests the secretion of hydrolytic enzymes into the medium. Although the crude enzyme extract—defined as the non-purified culture supernatant—was preliminarily tested on fresh CFM, this observation was qualitative and not quantitatively evaluated. Therefore, it is not presented as experimental evidence in this study. The viscosity reduction trends observed during fermentation remain the primary support for the enzymatic activity. The flow curves were well-fitted (
r2 ˃ 0.90) by Herschel-Bulkley equation (
).
. Flow curve of cladode flour medium (20%) for (<strong>a</strong>) <em>A. pittii </em>and (<strong>b</strong>) <em>B. bacillus</em> growth at different times (0 h, upper black circles; 24 h, black circles; 96 h, dark grey circles; and 168 h, light grey circles) with. Experimental curves: upward (increasing shear rate), close symbols, and downward (decreasing shear rate), open symbols. Modelling curves (upward: continuous line, downward: dashed line). Average of two replicates.
The ascending and descending curves of
A. pittii and
B. subtilis in CFM obtained during growth time (0, 24, 96 and 168 h) showed that yield stress (τ
0), viscosity (h) and consistency coefficient (K) decreased as a function of time, whereas the flow behavior index values (n) were lower when τ🡪0, and increased with growth time (
).
.
Rheological parameters (τ0, K and η) of upward curves a of cladode flour medium (20%) added with hydrolytic bacteria as a function of growth time.
| Strain |
Time (h) |
τ0 (Pa) |
K (cP) |
n (Dimensionless) |
η b (Pa·s) |
r2 |
|
| Control c |
0 |
261 ± 23 |
49 ± 16 |
0.20 ± 0.13 |
1.42 ± 0.20 |
0.934 |
| 24 |
256 ± 16 |
59 ± 12 |
0.22 ± 0.01 |
2.15 ± 0.05 |
0.955 |
| 96 |
362 ± 98 |
50 ± 6 |
0.22 ± 0.03 |
3.32 ± 0.01 |
0.972 |
| 168 |
248 ± 64 |
44 ± 1 |
0.24 ± 0.01 |
2.25 ± 0.01 |
0.927 |
| A. pittii |
0 |
352 ± 151 |
67 ± 45 |
0.35 ± 0.03 |
2.09 ± 0.70 |
0.965 |
| 24 |
269 ± 32 |
37 ± 8 |
0.56 ± 0.03 |
1.80 ± 0.12 |
0.955 |
| 96 |
175 ± 25 |
62 ± 9 |
0.53 ± 0.02 |
1.30 ± 0.59 |
0.937 |
| 168 |
70 ± 17 |
33 ± 18 |
0.44 ± 0.06 |
0.73 ± 0.11 |
0.956 |
| B. subtilis |
0 |
280 ± 16 |
45 ± 6 |
0.54 ± 0.02 |
1.90 ± 0.05 |
0.94 |
| 24 |
298 ± 46 |
31 ± 11 |
0.52 ± 0.09 |
1.39 ± 0.15 |
0.067 |
| 96 |
149 ± 31 |
46 ± 14 |
0.47 ± 0.02 |
1.30 ± 0.35 |
0.962 |
| 168 |
76 ± 10 |
35 ± 1 |
0.48 ± 0.01 |
0.81 ± 0.04 |
0.958 |
Flow index values obtained for
A. pittii and
B. subtilis (0.56 ± 0.03 and 0.54 ± 0.02, respectively) were higher compared to the control (culture medium without microorganisms) (0.22 ± 0.01) indicating a higher fluidity and a loss of the pseudoplasticity of CFM and therefore, a degradation of the raw material by the hydrolytic bacteria. Statistical analysis indicated that there were significant differences in flow index due to strain, time and the interaction of both factors (
).
.
Probability of individual and interaction effects on studied factors (strain and time) on the rheological parameters of cladode flour medium.
| Factors |
p-Values of Independent Variables |
| τ0 (Pa) |
K (cP) |
η (Dimensionless) |
Η a (cP) |
| Individual factors |
| A: Strain |
<0.001 b |
0.090 |
<0.001 b |
<0.001 b |
| B: Time |
<0.001 b |
<0.001 b |
<0.001 b |
<0.001 b |
| Interaction |
| AxB |
<0.001 b |
0.316 |
<0.001 b |
<0.001 b |
Both model curves (upward and downward) showed that CFM with
A. pittii and
B. subtilis exhibited a close to zero thixotropic area despite its high-water absorption capacity due to the hydrocolloid nature (30 g/100 g of mucilage).
4. Discussion
The fermentation of
Opuntia ficus-indica cladode flour medium (CFM) by native hydrolytic bacteria led to significant modifications in the physicochemical properties of this system, particularly in the reduction of viscosity and the development of a pseudoplastic flow profile. Consistent with our findings, recent studies have reported that enzymatic hydrolysis during fermentation significantly decreases the viscosity of the medium. For instance, Song et al. (2022) [
48] observed very low viscosities (~14.5 cP) in
Opuntia humifusa extracts fermented with cellulolytic enzymes in contrast to considerably higher viscosities of the unfermented controls. This effect reflects the well-established pseudoplastic nature of
Opuntia mucilage: a viscous biopolymer that exhibits shear-thinning behavior, with a flow index n < 1 that becomes more pronounced at higher concentrations. The enzymatic activity of
Bacillus subtilis and
Acinetobacter pittii, which reached maximum specific hydrolytic activities (SHA) of 0.22 ± 0.01 IU and 0.21 ± 0.05 IU, respectively, at 24 h, revealed a pivotal role in the hydrolysis of polysaccharides present in the medium. In particular,
Bacillus subtilis is a robust cellulolytic bacterium; Malik and Javed (2021) [
49] reported that
B. subtilis strains can produce high levels of cellulases (e.g., CMCase ≈ 2.4 IU/mL) capable of degrading lignocellulose. Similarly,
Acinetobacter pittii, isolated from decomposing cladodes, exhibited strong cellulolytic activity on cactus tissues. In a previous study, López-Domínguez et al. (2019) [
50] reported up to 0.67 IU/mL of total cellulase activity (FPase) and 0.23 IU/mL of endoglucanase activity within 24 h when using
A. pittii in a 20% cladode suspension.
The reduction in viscosity (~60%) observed after fermentation of the cactus flour suspension may be attributed to hydrolytic activity targeting the fiber polysaccharides. While this aligns with reports on microbial degradation of mucilage, our study specifically confirmed enzymatic activity using carboxymethyl cellulose (CMC) as a model substrate.
Although both bacterial strains exhibited similar SHA values, the progression of enzymatic activity over time revealed differences in metabolic dynamics, with
B. subtilis achieving higher early-stage activity, which may be advantageous for short-cycle bioprocesses. The sustained activity observed up to 48 h suggests a stable production of hydrolytic enzymes, compatible with longer fermentation periods. The enzyme activity data correlate with the 60% reduction in viscosity of the 20% cladode flour suspension, supporting the hypothesis that enzymatic breakdown of fiber and mucilage leads to structural disintegration of the medium. In line with previous studies where
Bacillus subtilis strains exhibited both amylolytic and cellulolytic activity during the microbial saccharification of plant-based residues [
51], the present work highlights the applicability of native strains for enzymatic degradation and biomass valorization of complex substrates.
The composition of
Opuntia cladodes, rich in mucilage, insoluble fiber, and calcium-bound polysaccharides, influences the substrate’s susceptibility to hydrolysis. The acidity and pH of cladodes are partially determined by the presence of organic acids generated via crassulacean acid metabolism (CAM), an adaptation of cacti to arid environments that also affects the fermentability of the substrate [
33]. Recent studies indicate that water retention in fiber-rich flours is governed mainly by branched, highly polar polysaccharides (e.g., pectins, hemicelluloses) rather than by cellulose or lignin [
38,
52]. These non-cellulosic fibers possess abundant hydroxyl and carboxyl groups, conferring much higher water-binding capacity than the crystalline cellulose or relatively hydrophobic lignin [
52]. In
Opuntia cladode flour, the native mucilage—a high-molecular-weight heteropolysaccharide rich in galacturonic acid and neutral sugars—chelates Ca²⁺ to form extensive cross-linked, gel-like networks that trap water and impart high viscosity [
47,
53]. When microbial cell wall hydrolases (e.g., cellulases, pectinases, hemicellulases) are applied, these polysaccharide matrices are cleaved and the previously bound water is released, leading to a dramatic loss of viscosity and altered rheological behavior [
5]. In the present study, although only endoglucanase activity was directly evaluated using CMC as a substrate, a comparable decrease in viscosity was observed, suggesting that similar hydrolytic mechanisms may be involved. However, the presence of pectinolytic or hemicellulolytic activity remains a plausible hypothesis that warrants further investigation.
From a rheological standpoint, CFM displayed marked pseudoplastic behavior after bacterial fermentation, characterized by a decrease in the flow behavior index (n < 1). This non-Newtonian response is consistent with previous studies using enzymatic hydrolysis on plant-based suspensions. Kim et al. (2013) [
54], for instance, reported a 70% reduction in viscosity using Viscozyme on 20% cladode suspensions, confirming the effectiveness of multi-enzyme cocktails in degrading mucilage and cellulose. In this study, a comparable decrease in viscosity was achieved using crude supernatants from wild bacteria, suggesting their potential for fiber degradation. Although the enzymatic spectrum of the bacterial extracts was not fully characterized, the reduction was consistent with microbial action on polysaccharides.
The enzymatic performance observed, mainly reflected by viscosity loss and endoglucanase activity, suggests that pectin degradation might also occur, although no direct pectinolytic assays were performed. This hypothesis is supported by prior studies on microbial action over
Opuntia mucilage [
5,
6]. This process involves the cleavage of galacturonic acid-rich domains within the mucilage matrix, facilitating breakdown and access to underlying polysaccharides. Furthermore, the comparatively lower enzymatic activity detected in
Acinetobacter pittii relative to
A. anitratus [
55] may be attributed to the complex architecture of the culture medium based on cladode flour (CFM), where mucilage layers can sterically hinder access to glycosidic bonds within cellulose microfibrils [
56].
Finally, while the results are promising, variability in cladode composition depending on harvest season, maturity, and postharvest treatment could influence the reproducibility of enzymatic degradation. Future studies should address the standardization of the substrate and evaluate the scalability of bacterial fermentation to optimize hydrolytic enzyme production and polysaccharide breakdown. Nevertheless, this work demonstrates that
O. ficus-indica cladodes, in combination with native hydrolytic bacteria, represent a viable and sustainable option for biomass valorization through enzymatic pretreatment in the context of green biotechnologies.
5. Conclusions
Bacteria isolated from decayed cladodes were identified as Acinetobacter pittii and Bacillus subtilis. Both had a maximum hydrolytic activity on a culture medium based on cladodes flour during the first 24 h, with no significant difference. All samples presented non-Newtonian behavior, and the upward and downward curves were well fitted to Herschel-Bulkley equation. In the culture medium, the consistency (K) decreased and presented a loss of pseudoplasticity (n) with the addition of microorganisms and growth time, concluding that the raw material was degraded positively by the effect of extracellular enzymes. Statistically, it was shown that the interaction of both factors (type of strain and time) had a significant influence. Although specific enzymatic pathways were not characterized, the observed hydrolytic trends and sugar release suggest the action of extracellular enzymes. However, further studies are necessary to confirm the type and specificity of the enzymatic activities involved. Statistically, the interaction between strain type and incubation time had a significant influence on both flow behavior and sugar release. Considering the low total solids content of cladodes, future optimization strategies should explore pretreatment (e.g., drying or concentration) or co-substrate approaches to enhance fermentation efficiency and improve substrate availability in biotechnological applications.
Author Contributions
Conceptualization, M.O.R.-S., I.M.R.-B. and C.M.L.-D.; Methodology, I.M.R.-B. and C.M.L.-D.; Software, C.M.L.-D. and K.A.A.-B.; Validation, M.O.R.-S., I.M.R.-B. and C.M.L.-D.; Formal Analysis, M.O.R.-S. and I.M.R.-B; Investigation, I.M.R.-B. and K.A.A.-B.; Resources, M.O.R.-S.; Data Curation, C.M.L.-D.; Writing—Original Draft Preparation, M.O.R.-S. and C.M.L.-D.; Writing—Review & Editing, C.M.L.-D., K.A.A.-B. and I.M.R.-B.; Visualization, M.O.R.-S.; Supervision, M.O.R.-S., I.M.R.-B.; Project Administration, M.O.R.-S., I.M.R.-B.; Funding Acquisition, I.M.R.-B.
Ethics Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the National Council of Science and Technology of Mexico (CONACYT) and Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA) with project No. 195157. The author López-Domínguez thanks to National Council of Science and Technology of Mexico (CONACYT) for scholarship No. 265844.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1.
Prisa D. Opuntia plants nutritive and medicinal value: A review.
J. Curr. Sci. Technol. 2023,
13, 486–499. doi:10.59796/jcst.V13N2.2023.1762.
[Google Scholar]
2.
SIAP. Servicio de Información Agroalimentaria y Pesquera. Available online: http://www.gob.mx/siap/ (accessed on 10 May 2024).
3.
Bensadón S, Hervert-Hernández D, Sáyago-Ayerdi SG, Goñi I. By-products of
Opuntia ficus-indica as a source of antioxidant dietary fiber.
Plant Foods Hum. Nutr. 2010,
65, 210–216. doi:10.1007/s11130-010-0176-2.
[Google Scholar]
4.
SAGARPA. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Available online: https://www.gob.mx/sagarpa (accessed on 13 March 2016).
5.
Bermejo LA, Sánchez-Zapata E, Pérez-Álvarez JA, Viuda-Martos M. Effect of enzymatic treatment on dietary fiber structure and functional properties in cactus pear (
Opuntia ficus-indica) peel.
Food Hydrocoll. 2022,
129, 107662. doi:10.1016/j.foodhyd.2022.107662.
[Google Scholar]
6.
Garfias Silva V, Cordova Aguilar MS, Ascanio G, Aguayo JP, Pérez-Salas KY, Susunaga Notario AC. Acid hydrolysis of pectin and mucilage from cactus (
Opuntia ficus) for identification and quantification of monosaccharides.
Molecules 2022,
27, 5830. doi:10.3390/molecules27185830.
[Google Scholar]
7.
Rodríguez-González F, Pérez-González J, Muñoz-López CN, Vargas-Solano SV, Marín-Santibáñez BM. Influence of age on molecular characteristics and rheological behavior of nopal mucilage.
Food Sci. Nutr. 2021,
9, 6776–6785. doi:10.1002/fsn3.2629.
[Google Scholar]
8.
Luna-Zapién EA, Zegbe JA, Meza-Velázquez JA, Contreras-Esquivel JC, Morales-Martínez TK. Mucilage yield, composition, and physicochemical properties of cultivated cactus pear varieties as influenced by irrigation.
Agronomy 2023,
13, 419. doi:10.3390/agronomy13020419.
[Google Scholar]
9.
Martins M, Ribeiro MH, Almeida CMM. Physicochemical, nutritional, and medicinal properties of
Opuntia ficus-indica (L.) Mill. and its main agro-industrial use: A review.
Plants 2023,
12, 1512. doi:10.3390/plants12071512.
[Google Scholar]
10.
Quintero-García M, Gutiérrez-Cortez E, Bah M, Rojas-Molina A, Cornejo-Villegas MA, Real AD, Rojas-Molina I. Comparative analysis of the chemical composition and physicochemical properties of the mucilage extracted from fresh and dehydrated
Opuntia ficus-indica cladodes.
Foods 2021,
10, 2137. doi:10.3390/foods10092137.
[Google Scholar]
11.
León-Martínez FM, Méndez-Lagunas LL, Rodríguez-Ramírez J. Spray drying of nopal mucilage (
Opuntia ficus-indica): Effects on powder properties and characterization.
Carbohydr. Polym. 2010,
81, 864–870. doi:10.1016/j.carbpol.2010.03.061.
[Google Scholar]
12.
Sáenz C, Sepúlveda E, Pak N, Lecaros M. Chemical and physical characterization of cactus cladodes (
Opuntia ficus-indica) powder.
Ital. J. Food Sci. 2010,
22, 416–423.
[Google Scholar]
13.
Medina-Torres L, Brito-De La Fuente E, Torrestiana-Sanchez B, Alonso S. Mechanical properties of gels formed by mixtures of mucilage gum (
Opuntia ficus-indica) and carrageenans.
Carbohydr. Polym. 2003,
52, 143–150. doi:10.1016/S0144-8617(02)00269-2.
[Google Scholar]
14.
Rooyen B, de Wit M, Osthoff G, van Niekerk J. Cactus pear mucilage (
Opuntia spp.) as a novel functional biopolymer: Mucilage extraction, rheology and biofilm development.
Polymers 2024,
16, 1993. doi:10.3390/polym16141993.
[Google Scholar]
15.
Cai W, Gu X, Tang J. Extraction, purification, and characterization of the polysaccharides from
Opuntia milpa alta.
Carbohydr. Polym. 2008,
71, 403–410. doi:10.1016/j.carbpol.2007.06.008.
[Google Scholar]
16.
Fernández-Martínez MC, Jiménez-Martínez C, Jaime-Fonseca MR, Alamilla-Beltrán L. Extraction of purple prickly pear (
Opuntia ficus-indica) mucilage by microfiltration, composition, and physicochemical characteristics.
Polymers 2024,
16, 3383. doi:10.3390/polym16233383.
[Google Scholar]
17.
Feugang JM, Konarski P, Zou D. Nutritional and medicinal use of cactus pear (
Opuntia spp.) cladodes and fruits.
Front Biosci. 2006,
11, 2574–2589.
[Google Scholar]
18.
Gurrieri S, Miceli L, Lanza CM, Tomaselli F, Bonomo RP, Rizzarelli E. Chemical characterization of Sicilian prickly pear (
Opuntia ficus-indica) and perspectives for the storage of its juice.
J. Agric. Food Chem. 2000,
48, 5424–5431. doi:10.1021/jf9907844.
[Google Scholar]
19.
Atlas R. Handbook of Microbiological Media, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2004.
20.
AOAC. Official Methods of Analysis, 14th ed.; AOAC: Washington, DC, USA, 1984.
21.
AOAC. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005.
22.
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances.
Anal. Chem. 1956,
28, 350–356.
[Google Scholar]
23.
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar.
Anal. Chem. 1959,
31, 426–428. doi:10.1021/ac60147a030.
[Google Scholar]
24.
United States Environmental Protection Agency (EPA). Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) (Revision 4.4); U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1994.
25.
Ayadi MA, Abdelmaksoud W, Ennouri M, Attia H. Cladodes from
Opuntia ficus-indica as a source of dietary fiber: Effect on dough characteristics and cake making.
Ind. Crops Prod. 2009,
30, 40–47. doi:10.1016/j.indcrop.2009.01.003.
[Google Scholar]
26.
Teather RM, Wood PJ. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen.
Appl. Environ. Microbiol. 1982,
43, 777–780. doi:10.1128/aem.43.4.777-780.1982.
[Google Scholar]
27.
Bartholomew JW, Finkelstein H. Relationship of cell wall staining to gram differentiation.
J. Bacteriol. 1958,
75, 77–84.
[Google Scholar]
28.
Hills PN, van Staden J. An improved DNA extraction procedure for plant tissues with a high phenolic content.
S. Afr. J. Bot. 2002,
68, 549–550. doi:10.1016/S0254-6299(15)30384-7.
[Google Scholar]
29.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data.
Bioinformatics 2012,
28, 1647–1649. doi:10.1093/bioinformatics/bts199.
[Google Scholar]
30.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool.
J. Mol. Biol. 1990,
215, 403–410. doi:10.1016/S0022-2836(05)80360-2.
[Google Scholar]
31.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.
Anal. Biochem. 1976,
72, 248–254. doi:10.1016/0003-2697(76)90527-3.
[Google Scholar]
32.
Ramírez F, Demirer G, Gallegos C, Hernández G, Santoyo V, Espinosa T. Life cycle assessment of biogas production through anaerobic co-digestion of nopal cladodes and dairy cow manure.
J. Clean Prod. 2018,
172, 2313–2322.
[Google Scholar]
33.
Astello-García MG, Cervantes I, Nair V, Santos-Díaz MS, Reyes-Agüero A, Guéraud F, et al. Chemical composition and phenolic compounds profile of cladodes from
Opuntia spp. cultivars with different domestication gradient.
J. Food Compos. Anal. 2015,
43, 119–130. doi:10.1016/j.jfca.2015.04.016.
[Google Scholar]
34.
Blanco-Macías F, Magallanes-Quintanar R, Valdez-Cepeda RD, Vázquez-Alvarado R, Olivares-Sáenz E, Gutiérrez-Ornelas E, Murillo-Amador B. Nutritional reference values for
Opuntia ficus-indica determined by means of the boundary-line approach.
J. Plant Nutr. Soil Sci. 2010,
173, 927–934. doi:10.1002/jpln.200900147.
[Google Scholar]
35.
Rodríguez-García ME, De Lira C, Hernández-Becerra E, Cornejo-Villegas MA, Palacios-Fonseca AJ, Rojas-Molina I, Muñoz-Torres C. Physicochemical characterization of nopal pads (
Opuntia ficus-indica) and dry vacuum nopal powders as a function of the maturation.
Plant Foods Hum. Nutr. 2007,
62, 107–112. doi:10.1007/s11130-007-0049-5.
[Google Scholar]
36.
Rojas-Molina I, Gutiérrez-Cortez E, Bah M, Rojas-Molina A, Ibarra-Alvarado C, Rivera-Muñoz E, Aguilera-Barreiro MDLA. Characterization of calcium compounds in
Opuntia ficus-indica as a source of calcium for human diet.
J. Chem. 2015,
2015, 710328. doi:10.1155/2015/710328.
[Google Scholar]
37.
Sáenz C. Cladodes: a source of dietary fiber.
J. Prof. Assoc. Cactus. Dev. 1997,
2, 117–123.
[Google Scholar]
38.
Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications.
Food Chem. 2011,
124, 411–421. doi:10.1016/j.foodchem.2010.06.077.
[Google Scholar]
39.
López-Cervantes J, Sánchez‑Machado DI, Campas‑Baypoli ON, Sánchez‑Duarte RG. Chemical composition and physicochemical properties of nopal (
Opuntia ficus‑indica) flour.
Food Sci. Technol. 2011,
31, 676–682. doi:10.1590/S0101-20612011000300016.
[Google Scholar]
40.
Shoukat R, Cappai M, Pia G, Pilia L. An Updated Review:
Opuntia ficus indica (OFI) Chemistry and Its Diverse Applications.
Appl. Sci. 2023,
13, 7724. doi:10.3390/app13137724.
[Google Scholar]
41.
Pourramezan Z, Ghezelbash GR, Romani B, Ziaei S, Hedayatkhah A. Screening and identification of newly isolated cellulose-degrading bacteria from the gut of xylophagous termite
Microcerotermes diversus (Silvestri).
Mikrobiologiia 2012,
81, 796–802. doi:10.1134/S0026261712060124.
[Google Scholar]
42.
Rasi RPM, Mahalingam PU. Screening and partial characterization of cellulose degrading bacteria from decayed sawdust.
Int. J. Sci. Res. 2014,
3, 957–960.
[Google Scholar]
43.
Bernabé-Salomon LA. Identificación molecular de bacterias cultivadas y no cultivadas asociadas a la rizósfera de Opuntia ficus-indica (L.) Mill. (Cactaceae) en ecosistemas áridos; Bachelor Thesis, Universidad Nacional de Tumbes, Tumbes, Perú, 2016.
44.
Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century.
3 Biotech. 2016,
6, 174. doi:10.1007/s13205-016-0485-8.
[Google Scholar]
45.
Dhiman SS, Sharma J, Battan B. Industrial applications and future prospects of microbial xylanases: a review.
BioResources 2008,
3, 1377–1402.
[Google Scholar]
46.
Béguin P, Aubert JP. The biological degradation of cellulose.
FEMS Microbiol. Rev. 1994,
13, 25–58. doi:10.1111/j.1574-6976.1994.tb00033.x.
[Google Scholar]
47.
Medina-Torres L, Brito-De La Fuente E, Torrestiana-Sanchez B, Katthain R. Rheological properties of the mucilage gum (
Opuntia ficus-indica).
Food Hydrocoll. 2000,
14, 417–424. doi:10.1016/S0268-005X(00)00015-1.
[Google Scholar]
48.
Song W, Lagmay VQ, Jeong B-G, Jung J. Changes in physicochemical and functional properties of
Opuntia humifusa during fermentation with cellulolytic enzyme and lactic acid bacteria.
LWT-Food Sci. Technol. 2022,
159, 113192. doi:10.1016/j.lwt.2022.113192.
[Google Scholar]
49.
Malik WA, Javed S. Biochemical characterization of cellulase from
Bacillus subtilis strain CD001 and its effect on digestibility and structural modifications of lignocellulose-rich biomass.
Front. Bioeng. Biotechnol. 2021,
9, 800265. doi:10.3389/fbioe.2021.800265.
[Google Scholar]
50.
López-Domínguez CM, Ramírez-Sucre MO, Rodríguez-Buenfil IM. Enzymatic hydrolysis of
Opuntia ficus-indica cladode by
Acinetobacter pittii and alcohol fermentation by
Kluyveromyces marxianus: pH, temperature and microorganism effect.
Biotechnol. Rep. 2019,
24, e00384. doi:10.1016/j.btre.2019.e00384.
[Google Scholar]
51.
Mushtaq Q, Ishtiaq U, Joly N, Spalletta A, Martin P. Harnessing
Bacillus subtilis QY5 PP784163 for bioethanol production from potato peel waste and nutrient recovery for animal feed: Maximizing resource efficiency.
Fermentation 2024,
10, 523. doi:10.3390/fermentation10100523.
[Google Scholar]
52.
Boulos N N, Greenfield H, Wills RBH. Water holding capacity of selected soluble and insoluble dietary fibre.
Int. J. Food Properties. 2000,
3, 231–244. doi:10.1080/10942910009524629.
[Google Scholar]
53.
Habibi Y, Mahrouz M, Vignon MR. Structural features of pectic polysaccharides from
Opuntia ficus-indica cladodes.
Carbohydr. Res. 2005,
340, 695–699. doi:10.1016/j.carres.2004.11.004.
[Google Scholar]
54.
Kim J, Lee H, Park Y, Ra K, Shin K, Yu K, Suh H. Mucilage removal from cactus cladodes (
Opuntia humifusa Raf.) by enzymatic treatment to improve extraction efficiency and radical scavenging activity.
LWT-Food Sci. Technol. 2013,
51, 337–342.
[Google Scholar]
55.
Ekperigin MM. Preliminary studies of cellulase production by
Acinetobacter anitratus and
Branhamella sp.
Afr. J. Biotechnol. 2007,
6, 28–33.
[Google Scholar]
56.
Bayar N, Friji M, Kammoun R. Optimization of enzymatic extraction of pectin from
Opuntia ficus-indica cladodes after mucilage removal.
Food Chem. 2018,
241, 127–134. doi:10.1016/j.foodchem.2017.08.051.
[Google Scholar]