Found 24 results
Open Access
Review
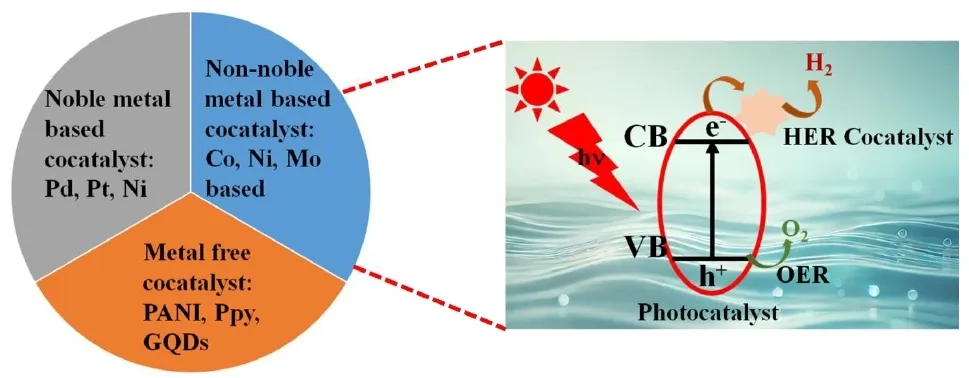
07 March 2025Unravelling the Role of Hydrogen Evolution Reaction Co-Catalysts in Photocatalytic Water Splitting: Mechanistic Insights and Material Strategies
The reliance on fossil fuels has led to a substantial increase in greenhouse gas emissions, presenting a critical environmental challenge. Addressing this issue necessitates the adoption of alternative renewable energy sources, with green hydrogen emerging as a promising candidate due to its high gravimetric energy density and absence of harmful emissions. Among the various hydrogen production techniques, photocatalytic technology has garnered significant attention for its dual potential to produce green hydrogen and degrade pollutants, thereby addressing both energy and climate crises. Efforts to scale photocatalytic technology for industrial applications have identified cocatalyst integration as a pivotal strategy, as it enhances reaction kinetics by lowering the activation energy and mitigating charge carrier recombination. This review comprehensively examines the hydrogen economy, the underlying principles of photocatalysis, recent technological advancements, key factors influencing photocatalytic reactions, the role of catalysts in hydrogen evolution reaction (HER) surface mechanisms, strategies for cocatalyst optimization, and future directions for the field.
Open Access
Article
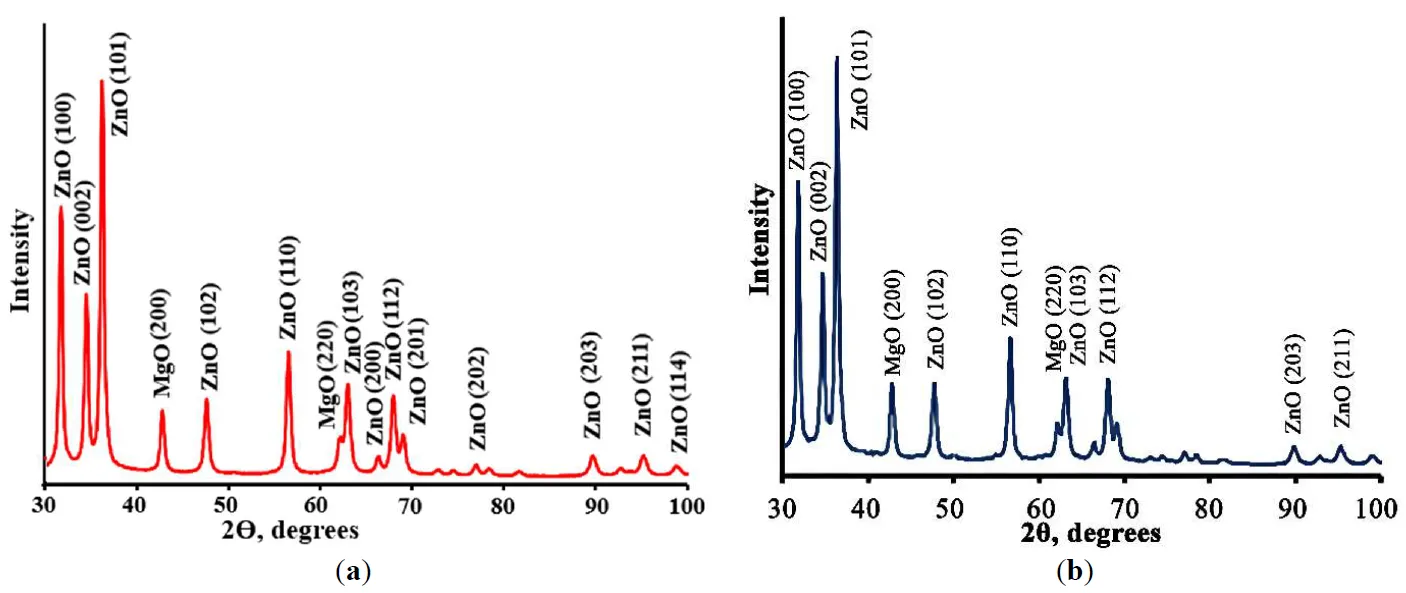
21 February 2025Porous Cu(Mn)-Doped ZnO-MgO Nanocomposites for Photocatalytic and Antibacterial Applications
Porous Cu(Mn):ZnO-MgO composites synthesized by polymeric sol-gel method were characterized. The crystal structure, morphology, spectral properties, the ability of the photogeneration of chemically active singlet oxygen under external visible irradiation, photocatalytic and antibacterial properties of porous composites were studied. Obtained composites consist of small ZnO and MgO crystals having size less than 20 nm. It was found that Cu2+ and Mn2+ ions are embedded into the lattices of ZnO and MgO crystals, altering their crystal cell parameters. The band gap values of obtained composites are 3.41 ÷ 3.42 eV which are slightly higher than the band gap of pure ZnO. Prepared materials demonstrate a high ability of photogeneration of chemically active singlet oxygen under blue light (λ = 405 nm) irradiation. It was found that dependencies of the intensity of singlet oxygen photogeneration from the power density of visible irradiation are linear. Photocatalytic decomposition of the diazo dye Chicago Sky Blue in solutions under UV and blue light irradiation proceeds rapidly in the presence of the prepared composites (constants rate of photocatalytic dye decomposition under UV irradiation are 0.024 min−1 and 0.025 min−1 for ZnO-MgO composites doped with Cu and Mn, correspondingly). Porous composites demonstrate superior antibacterial activity against gram-positive bacteria. These materials are promising for practical application in medicine and photocatalytic technologies of air and water cleaning.
Open Access
Article
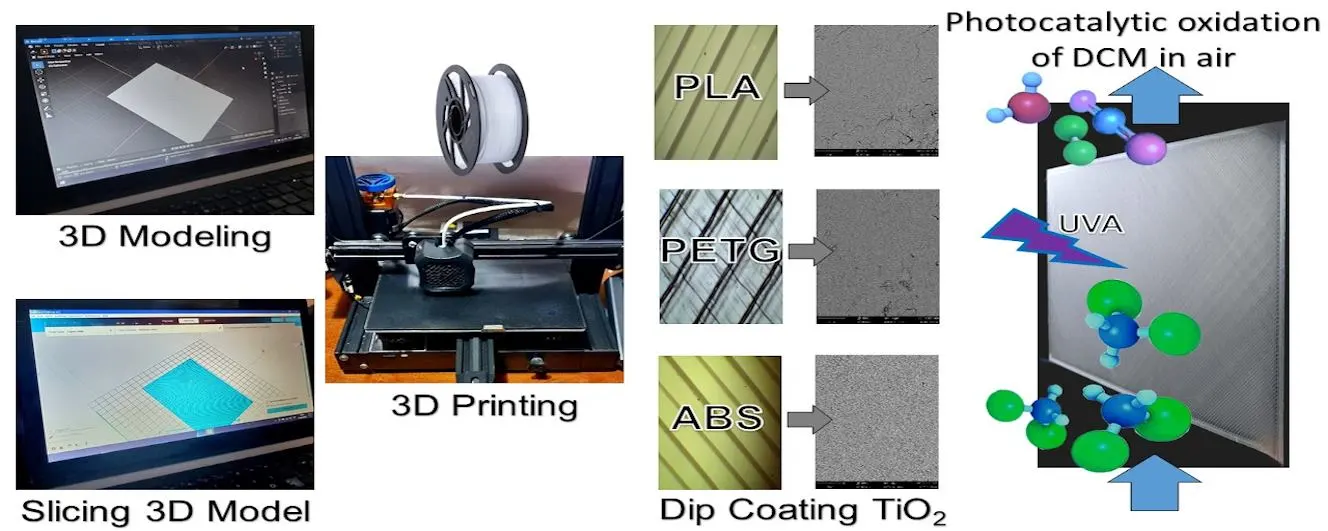
11 February 2025Functionalization of 3D-Printed Plastics for the Photocatalytic Removal of Organic Pollutants in Air
The study explored the use of 3D-printed plastics as catalyst supports for gas-phase photocatalytic applications. Specifically, it compared three commonly used plastic materials: PLA, ABS, and PETG. The process involved 3D modeling, additive manufacturing through 3D printing, and functionalization via dip-coating with titanium dioxide (TiO2). The study evaluated the loading capacity of the materials, the adhesion of the films, and the optical properties of the photocatalytic plates. Finally, the three plastic samples were tested as support materials in a laboratory-scale flat-plate reactor for the photocatalytic oxidation of dichloromethane in air. Loading capacities of around 3 mg/cm2 for TiO2 were achieved, along with radiation absorption capacities close to 65%. A correlation between loading and absorption fraction was identified, leading to the proposal of a simple saturation model; in turn, it allowed the predictive model of pollutant conversion as a function of the absorbed fraction of radiation. By analyzing both qualitative and quantitative properties and results, in order to determine the most suitable plastic material to be used in a photocatalytic wall reactor, PLA emerged as the best choice among the materials tested. These results show promise for the effective utilization of these plastics in the design of air decontamination devices.
Open Access
Review
14 October 2024Photocatalytic Antifouling Coating: From Fundamentals to Applications
With the rapid development of shipping industry, marine vessels frequently suffer from biofouling caused by marine organisms, making the effective prevention of marine biofouling a critical issue. Traditional antifouling coatings, which utilize toxic and harmful substances, pose significant risks to marine ecosystems. Therefore, the development of environmentally sustainable antifouling coatings has become imperative. Photocatalytic antifouling coatings, as an eco-friendly alternative, present a promising solution to these economic, energy, and ecological challenges. This review compares the environmental benefits of photocatalytic antifouling coatings to traditional ones, highlighting the underlying mechanisms of marine biofouling. Additionally, it explores the preparation techniques employed in photocatalytic antifoulant, analyzing the advantages, disadvantages, and potential modifications for photocatalytic coatings. Based on these insights, the future development of photocatalytic antifouling coatings is discussed, aiming to provide valuable references for the exploration of more efficient, broad-spectrum, energy-saving, environmentally friendly, and cost-effective marine antifouling technologies.
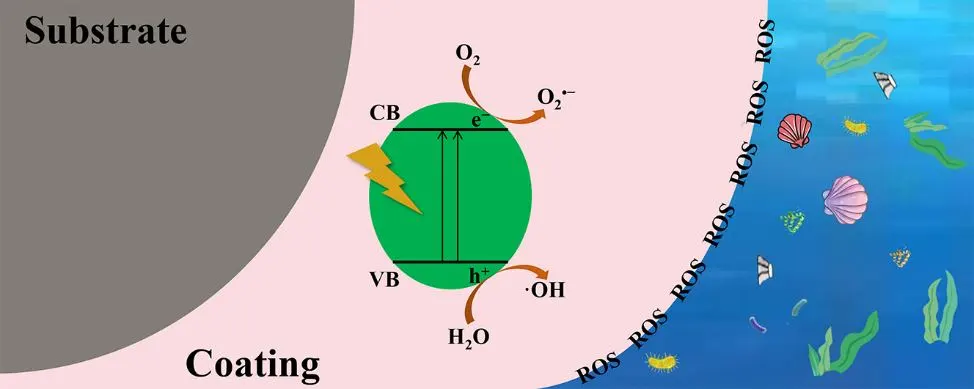
Open Access
Article
28 August 2024Photocatalytic and Photothermal Catalytic Oxidation of Ethene and Ethanol Using TiO2-Based Catalysts under UV-C and UV-A Irradiation
Photocatalytic (PCO) and photothermocatalytic oxidation (PTCO) of ethene (C2H4) and ethanol (EtOH) are investigated using TiO2 and 1%Pt/TiO2 coating on velvet glass support in the presence of UV-A and UV-C irradiation. Both VOC are efficiently mineralised under UV-A irradiation and PCO, but the presence of Pt has a minor impact on their transformation. Instead, there is only a slight increase in the disappearance of EtOH and the formation of acetaldehyde, which are already observed in the dark. Surprisingly, when a higher photon flux is emitted with a UV-C lamp, photocatalytic disappearance and mineralisation of EtOH are less effective than under UV-A irradiation in the presence or absence of Pt. Similar behaviour is also observed on C2H4 PCO in the presence of 1%Pt/TiO2 but not on its PCO mineralisation with TiO2, which is improved by a factor equivalent to the number of photons emitted. Under PTCO, by increasing the temperature from 40 °C to 120 °C, only a benefit impact is observed on C2H4 and EtOH disappearance but an important decrease of mineralization of C2H4 was observed in presence of TiO2 and UV-C The behaviour of these two VOCs under different irradiations and temperatures will be discussed according to the catalytic process.
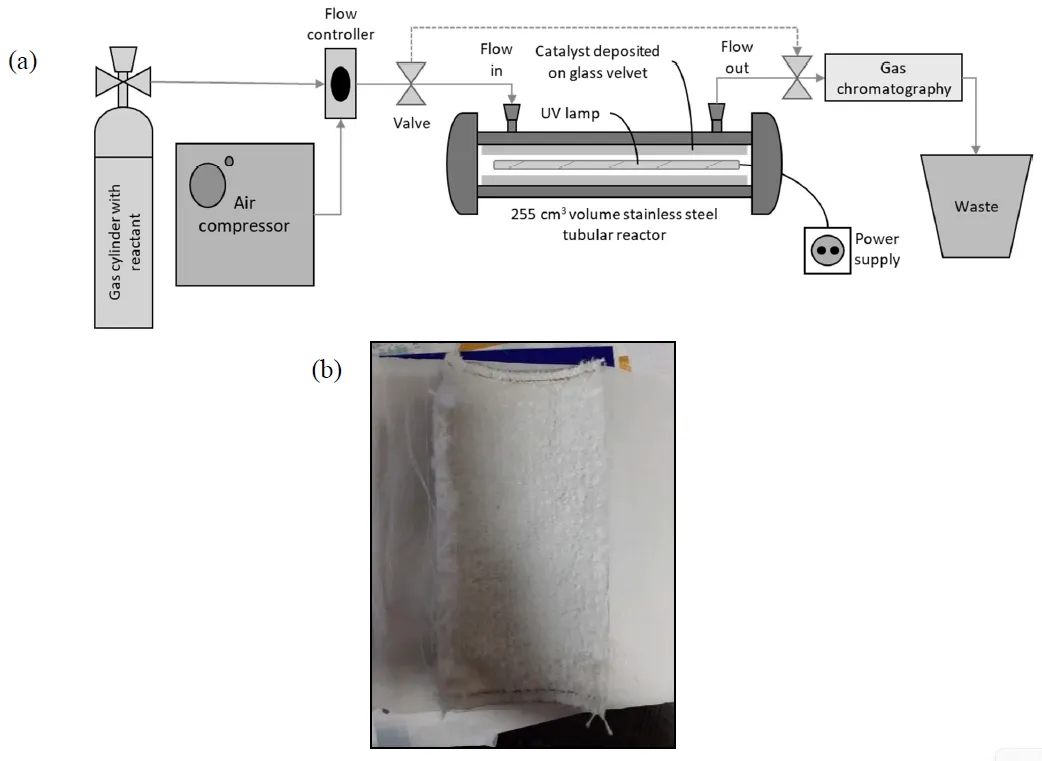
Open Access
Review
11 July 2024Photocatalytic Aerobic Conversion of Methane
The direct conversion of methane into high-value chemicals has been a persistent research focus in the fields of chemical engineering and energy. Photocatalysis, as an innovative technology, not only circumvents the issues of catalyst sintering and carbon deposition associated with traditional thermal catalysis but also transcends thermodynamic limitations by providing new reaction pathways. Utilizing molecular oxygen as an oxidant generates various reactive oxygen species, offering unique thermodynamic advantages for methane conversion. This review summarizes the advancements in photocatalytic partial oxidation (PPOM) and oxidative coupling of methane (POCM) using oxygen as an oxidant. It discusses the activation mechanisms and reaction pathways of methane and oxygen in different systems, as well as the application of photochemical cycling strategies in methane conversion. Finally, it addresses the challenges in this field, proposes potential solutions, and offers perspectives on the future development of photocatalytic systems.
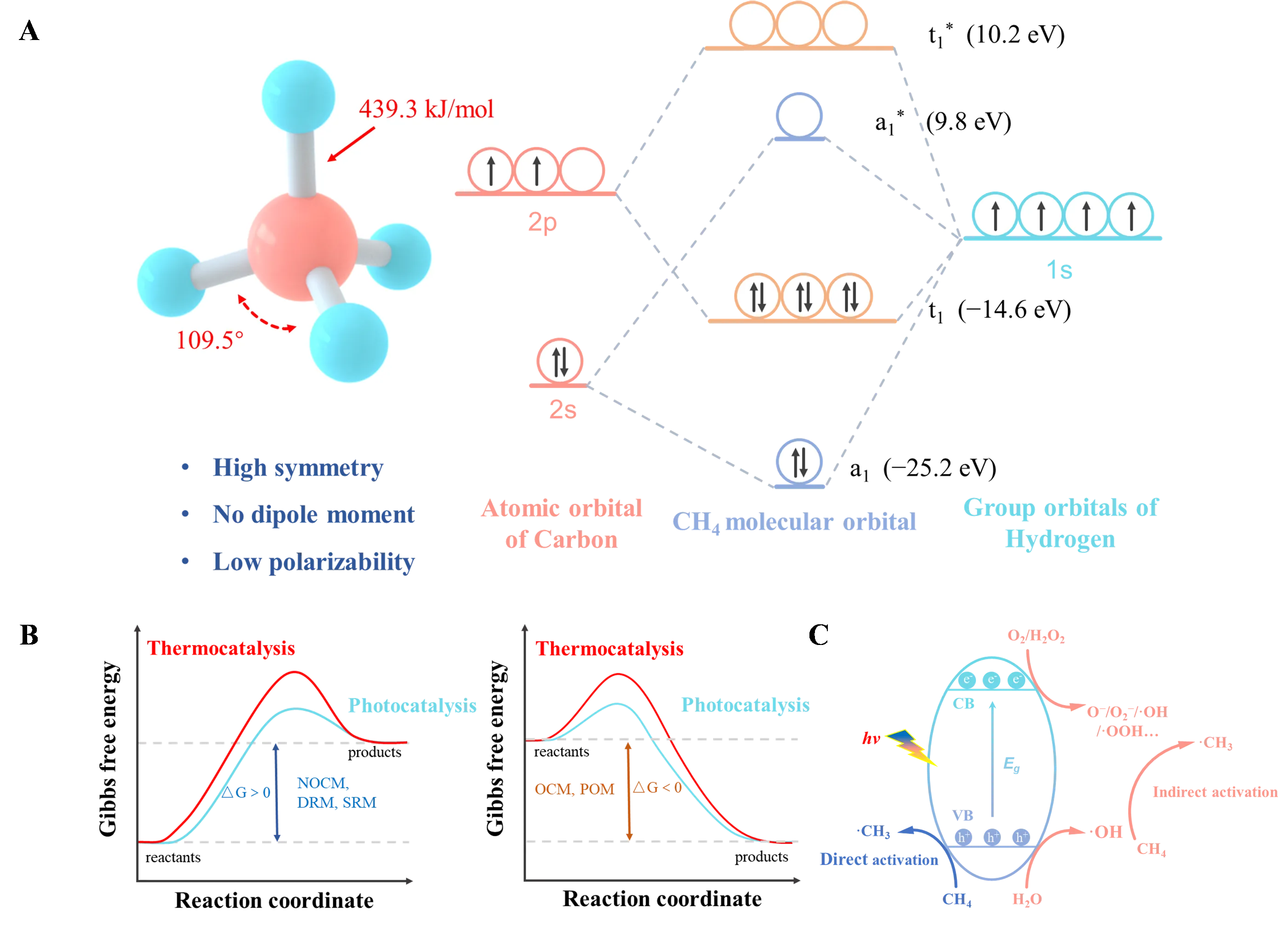
Open Access
Article
11 June 2024Lathyrus aphaca Extract MnO Nanoparticles: Synthesis, Characterization, and Photocatalytic Degradation of Methylene Blue Dye
Our environment has been impacted by man-made pollutants mainly industries make substantial use of synthetic dyes which exhibit cytotoxicity and have significant environmental consequences. Effective photocatalyst-based approaches for degrading synthetic dyes into less toxic chemical are of great interest. Synthesizing nanoparticles (NPs) using biological approaches, particularly plant-based approaches offer advantages, decreasing the risk of NPs losing biocompatibility during synthesis, cost-effectiveness, and eco-friendliness. In this study, we employed a green synthesis method to produce manganese oxide nanoparticles (MnO NPs) utilizing leaf extract from the Lathyrus aphaca plant. The synthesized MnOx NPs were characterized through various techniques; X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), Fourier-transform infrared spectroscopy (FTIR), and UV–visible spectroscopy. XRD analysis showed distinct peaks, indicated the presence crystallographic planes within the MnO2 nanoparticles, thus confirming their crystalline structure. FTIR, showed the presence of the O-O stretching mode at a frequency of 719 cm−1, the presence of MnO6 oxides of manganese, and peak at 548 cm−1 corresponded to the Mn-O stretching mode. Furthermore, the green-synthesized manganese oxide nanoparticles exhibited promising photocatalytic and adsorption capabilities against Methylene Blue (MB) dye, leading to approximately 93% degradation of MB when treated with the green-synthesized MnO nanoparticles derived from plant extract. This highlights the efficacy and potential of these nanoparticles in environmental remediation applications, particularly in the degradation of methylene blue contaminants.
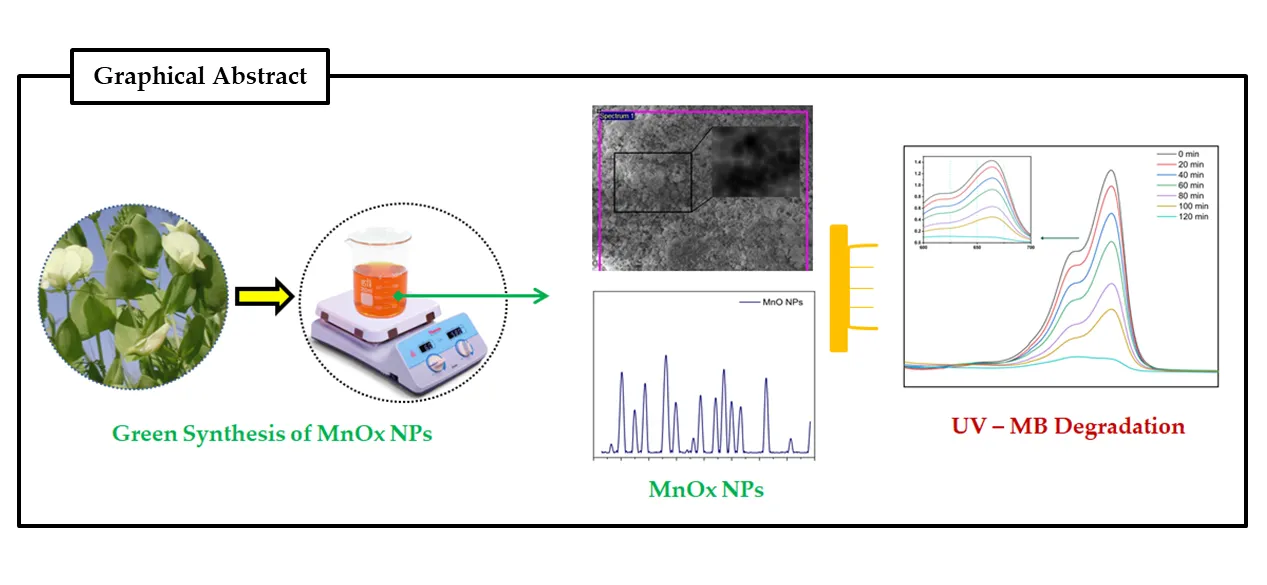
Open Access
Article
05 January 2024Benzene Bridged Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution
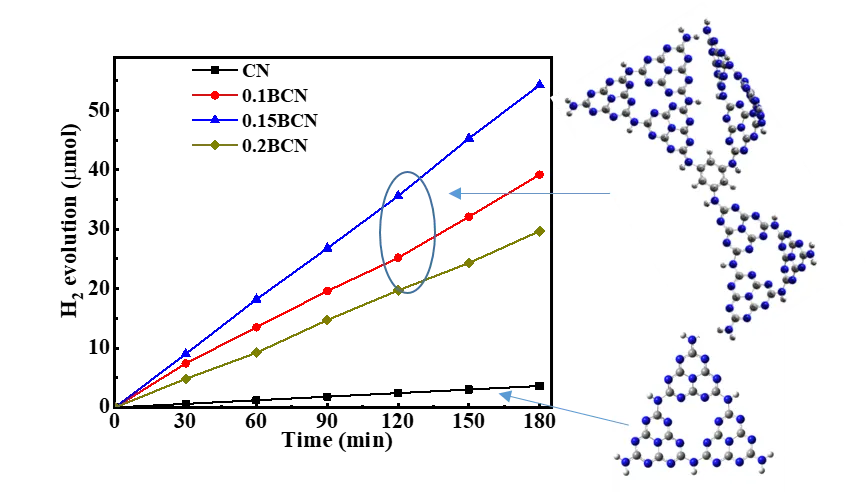
Turing the electronic structure by inserting certain functional groups in graphitic carbon nitride (g-C3N4, CN for short) skeleton through molecular doping is an effective way to improve its photocatalytic performance. Herein, we prepare a benzene bridged carbon nitride (BCN) by calcining urea and 1,3,5-tribromobenzene at elevated temperature. The introduction of benzene ring in g-C3N4 layers improves the separation efficiency and lifetime of photogenerated carriers, inhibits the recombination rate of electron/hole pairs, thus the performance of photocatalytic hydrogen evolution improves. The optimal hydrogen evolution rate of 1.5BCN reaches 1800 µmol/h·g, which is nine times that of the pure g-C3N4. DFT calculation proved the benzene bridged CN increased the distance of charge transfer (DCT) and the push-pull electronic effect of intramolecular electrons. This work may provide a pathway for preparing molecular doped g-C3N4 with improved photocatalytic performance.
Open Access
Article
11 October 2023Fibrous SiC-based Mesoporous Solids for the Photocatalytic Degradation of Organic Pollutants under Artificial Light
SiC-based mesoporous solids with fibrous nanostructure were prepared by impregnation of a polycarbosilane precursor on annealed polyacrylonitrile (PAN) fibers and subsequent pyrolysis. The obtained material exhibits a mesoporous structure and has a specific surface area of ~20 m2/g. It has a semiconducting electronic character with a bandgap of 2.65 eV, i.e., in the visible range. Adsorption tests of methylene blue were performed on the material under dark conditions, which showed an adsorption amount of 78 wt%. The photocatalytic activity of the material was then evaluated for the degradation of the dye under artificial daylight irradiation over a period of 7 h. A degradation of 94 wt% was achieved. Regeneration and reuse of the material was also tested and resulted in 97 wt% degradation after reuse, indicating potential interest of the material as a contactor in environmental remediation devices.
Open Access
Article
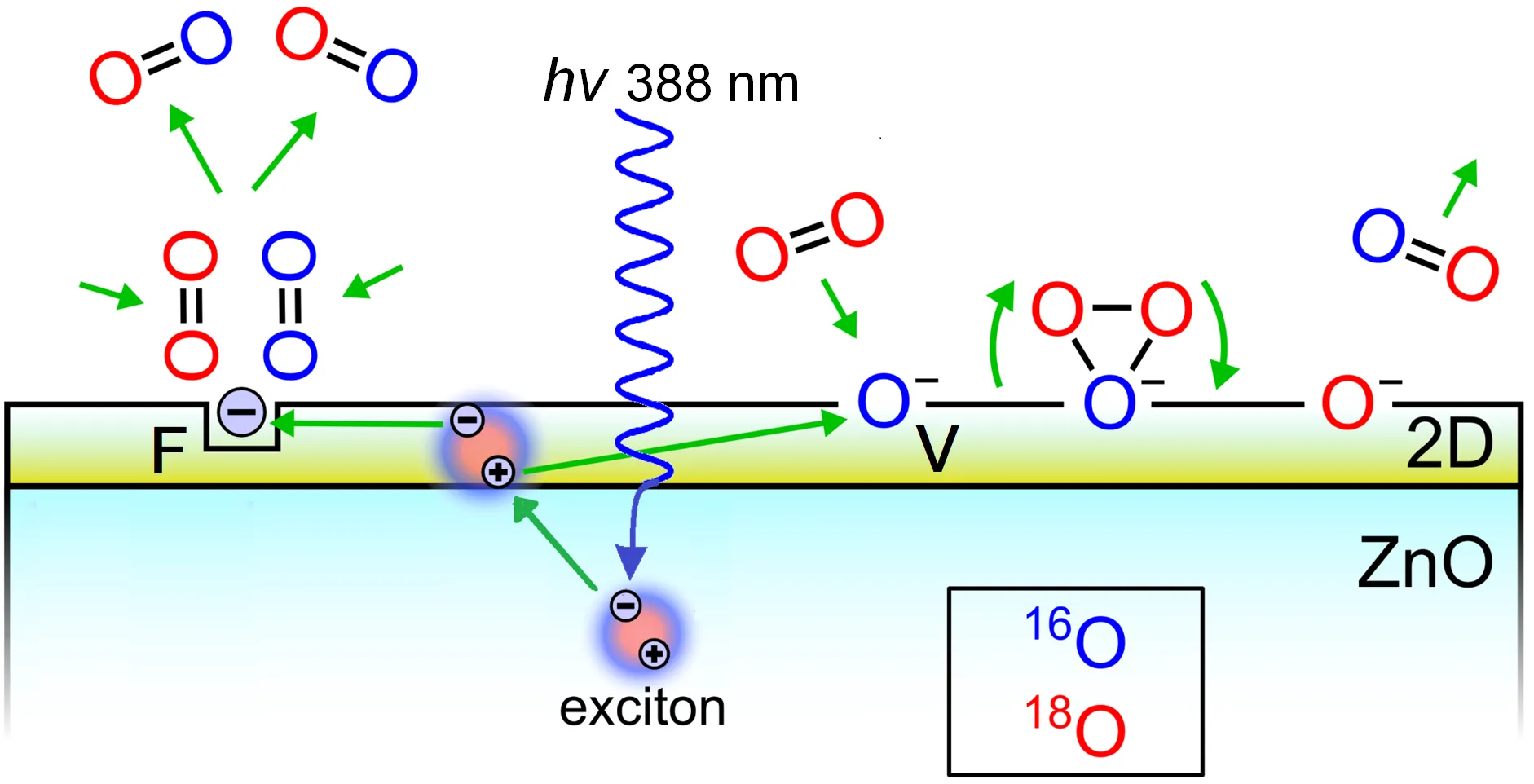
31 August 2023Potential Role of Exciton in Photocatalysis
This article commemorates the outstanding Russian scientists E.F. Gross and A.N. Terenin. It revisits their successors’ efforts to develop Terenin’s idea of using excitons, discovered by Gross, for photocatalytic redox reactions on wide-gap semiconductors. Terenin proposed ZnO as the subject of study. To explore the possibility of replacing photogenerated electrons and holes in a redox reaction by an exciton being a quasi-neutral particle, the test reaction of the photoactivated oxygen isotope exchange (POIE) was studied. It was found that many years of initial unsuccessful attempts were due to the fact that the exciton energy is spent on luminescence. In our experiments, the excitons decayed non-radiatively, and the long-lived electron-donor F-type and hole V-type active centers were formed by creating the 2D surface nanostructure ZnO/ZnO1−x/O−. These centers allowed to obtain the reaction efficiency 5–8 times higher than with the interband transitions. Thus, the developed 2D surface nanostructure ZnO/ZnO1−x/O− resolved the problem of using an exciton in photocatalysis and demonstrated the perspective of this nanostructure as an efficient photocatalyst.