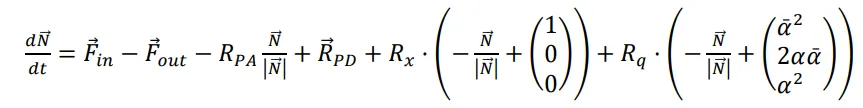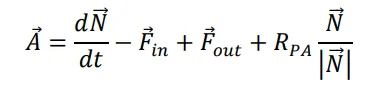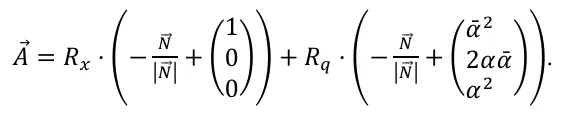In 2010s, the joint work of the successors of E.F. Gross and A.N. Terenin started. The planned program included 3 points:
1. Suppress the radiative decay of excitons on the surface.
2. Use the energy of nonradiative decay of an exciton to generate the long-lived electron-donor F-type and hole V-type surface centers.
3. Ensure the interaction of the generated centers with the molecules of the gas phase, resulting in the POIE reaction.
After an extended research, a way to suppress the channel of the radiative exciton decay was found. It was shown that the surface PL of ZnO can be suppressed by a specific change in oxygen concentration in the surface layer of the crystal and in the adsorbed layer [
9]. At the first stage, photo-desorption of adsorbed oxygen and dosed photo-reduction of the ZnO surface layer were carried out in Ultra High Vacuum (UHV). The amount of photo-desorbed oxygen was controlled by a mass spectrometer (MS) observing a decrease in the intensity of the subsequent thermo-desorption spectrum (TDS) of the adsorbed oxygen. The excess of photo-desorbed oxygen over the TDS can naturally be associated with the photo-desorption of surface structural oxygen. To confirm this, a 2D structure “core-shell” ZnO/ZnO
1−x was suggested to be formed. The value of “x” was determined by measuring the amount of released oxygen and the size of the illuminated surface [
9,
10]. A photo-processing caused changes in the electrical parameters of the sample (see ). According to measurements with an original ultra-violet photoelectron spectrometer (UPS, 8.43 eV) [
9,
11] from the outer side of the surface, the value of the dipole component Δδ of the thermoelectric work function φ
T dropped to zero. On the inner side of the surface, due to the recharging of “fast” surface states, the value of the band bending of the initial “oxidized” sample V
S decreased, and a potential barrier was created separating the surface states from the volume. The result of such processing was not a suppression, but an increase in the PL intensity compared to the original sample. The mechanism of the process was discussed in [
9].
The next step in the formation of the 2D structure was the adsorption of O
− radicals onto the surface. As a result, the barrier Δδ is restored on the outer side, forming a quantum well together with the inner barrier. Here ZnO/ZnO
1−x is a 2D structure “core-shell” and O
− is the adsorbed atomic oxygen capturing an electron, thus forming a locking barrier over the “core-shell” structure. A high field strength inside the well leads to a dissociative polarization of the exciton, causing its nonradiative decay. As a result, a “dead zone” for the exciton is created in the surface subatomic region of ZnO
1−x/O
−. The exciton decays, and the free electron and hole are localized on F- and V-type defects of the ZnO
1−x surface layer.
The 2D structure ZnO/ZnO
1−x/O
− was formed using ZnO high purity (99.99%) powder “OSCh 12-2” with the wurtzite structure, consisting of 20–500 nm nanocrystallites [
9]. The samples were characterized using optical and photo- luminescence spectroscopy (PL). Thermo-stimulated luminescence (TSL) in ultraviolet (UV) and visible (VIS) light, scanning electron microscopy (SEM), ultraviolet photoelectron spectroscopy (UPS) (21.4, 8.43 eV), and X-ray Diffraction (XRD). UPS (8.43 eV) allowed us to determine in situ the φ
Т of the sample and to separate the contributions of the dipole component δ from the band bending V
S. The 2D structure includes a sub-monolayer potential electron shell bounded from inside by the electron potential at the surface states and from outside by a potential barrier created by the adsorbed oxygen O
−.
We have studied this composite with original UPS (8.43 eV) and MS methods [
9,
10,
11]. The energy diagram of its 2D structure is shown in . In such a structure the exciton does not emit light, but decays into a pair
e− +
h+ which fills the vacancies appearing on the photo-restored surface.
. Radiationless decay of exciton into a pair of long-lived centers in the 2D structure ZnO/ZnO1−x/O−. VB—valence band, CB—conduction band, EF—Fermi level, VL—vacuum levels before and after oxygen desorption, Δδ—dipole component, φT—thermoelectric work function, VS—band bending of the original “oxidized” sample, A/A−, D/D+—acceptor and donor reagent levels.
The second point of the research program was to find out the fate of the electron and hole formed during the decay of the exciton. It is natural to expect the possibility of their localization on F- and V-type defects of the ZnO
1−x surface layer. To test this possibility, we studied TSL after illuminating the structure at the exciton absorption line. A TSL with a maximum at 475 K was detected (see ), comprising spectral regions typical for F- and V-type centers [
10]. The energy of the absorbed light was retained for 8 × 10
3 s, which is typical for F- and V-type centers. It is these centers that sensitize the photocatalytic activity of reduced ZnO
1−x samples [
12]. Thus, it has been shown that when the ZnO/ZnO
1−x/O
− structure is illuminated in the region of exciton resonance, the active centers are formed on its surface providing the photocatalytic activity of ZnO
1−x.
. Thermally stimulated luminescence (TSL) of F-type and V-type centers in ZnO/ZnO1−x /O− illuminated by a monochromatic light. Curve 1: λ = 365 nm, Curve 2: λ = 385 nm. Curve 3: λ = 385 nm, the sample preliminarily kept in an oxygen atmosphere at 10−1 Torr.
Finally, the third point of the program was to check whether the centers active on the ZnO
1−x surface will also be active on the surface of ZnO/ZnO
1−x/O
−. This can be verified by analyzing the interaction of active centers with oxygen molecules from the gas phase, since the criterion for the activity of a photo-catalyst is its activity in the POIE reaction. To characterize the interaction of gas molecules with a photo-activated surface, we analyzed the TDS of oxygen adsorbed onto ZnO/ZnO
1−x/O
−, which was previously exposed to the exciton absorption line. These TDS coincided with the previously obtained TDS of oxygen, adsorbed on the pre-illuminated ZnO
1−x photo-catalyst, and they had 3 maxima at E
des = 0.6, 1.1 and 1.3 eV. The first maximum is attributed to the desorption of O
2 from the $$\text{O}_2^-$$ form adsorbed on F-type electron-donor centers, the second one is attributed to the desorption of O
2 from the $$\text{O}_3^-$$ complex adsorbed on V-type hole centers, the third maximum is the result of the associative desorption of atomic oxygen.
Thus, it can be expected that the 2D structure ZnO/ZnO
1−x/O
− makes it possible to use the exciton energy to carry out the POIE reaction.
3.2. Experimental Study of the Photocatalytic Reaction of POIE on a 2D Structure ZnO/ZnO1−x/O−
In the experiments, two versions of the experimental flow-through reactor were used. The first one was made of optical quartz [
13]. The stainless steel chamber of the second reactor [
11] had two sapphire windows, one for UPS excitation light (146.6 nm, 8.43 eV) and another for UV-VIS photoexcitation of the sample and for thermo-PL detection. The continuous analysis of the gas composition above the sample was carried out on the first reactor at the pressures of 10
−1–10
−4 Torr, while on the second one at 10
−5–10
−8 Torr. The amount and the composition of the adsorbed layer was analyzed by the TDS method. A heater and a thermocouple allowed one to adjust and to control the sample temperature in the 77−750 K range.
The diagram of “flow-through” regime is presented in .
. Block diagram of the sample chamber.
The flow of O
2 gas
L0, injected into the chamber, passes over the sample, increasing by
L1 due to the desorption from the sample and decreasing by
L2 due to the adsorption on the sample, and the resulting flow
L0 +
L1 −
L2 enters the MS. The adsorption and desorption values measured as the deviations in the amplitudes of the MS signals from the stationary values
L0 correspond to the reaction rates (a differential measurement mode).
The electrostatic electron energy analyzer of original design [
11] is extremely compact and does not interfere with chemical measurements. The 99.99% chemical purity mixtures of isotopes
18O
2 and
16O
2 were used, while the ZnO sample had a natural isotopic composition. The POIE has been chosen as a test reaction which was successfully used [
4,
5,
6,
7,
8] to study the mechanism of photo-activation of heterogeneous reactions. Further details of the experimental setup and of the results processing are given in Ref. [
13].
For the interband excitation the 365 nm line was chosen. It was obtained from the DRSh-500 high-pressure Hg lamp using the LOMO glass color filters USF-6 and BS-7 and additionally accentuated by the KSVU-1 monochromator. For the exciton excitation, a RF-UVXC35LN-UD (Refond) UV LED was used. According to the datasheet, it should have a peak at 375−380 nm, but we measured it at 388 nm (). In the same figure, the light-emitting diode radiation spectrum multiplied by the absorption spectrum is presented. One can see that the spectrum of the absorbed radiation is situated inside the area of the exciton resonant excitation (). In both cases, the radiation power absorbed by the sample was (10 ± 2) mW.
. 1—spectra of the exciton resonant excitation at RT; 2—spectrum of the sample illumination by LED RF-UVXC35LN-UD (Refond), taking into account the absorption of the sample; 3—365 nm emission of DRSh-500 accentuated by the monochromator KSVU-1.
In our experiments we monitored the current concentrations (partial pressures) of
16O
2,
16O
18O and
18O
2 molecules in the gas phase as well as the total pressure above the illuminated sample. The obtained results presented in clearly show the presence of the photo-activation effect in the O
2–ZnO/ZnO
1−x/O
− system. For the quantitative analysis of the mechanism of the process, we have examined its individual stages.
. Effect of interband and exciton irradiation of the sample on the isotopic composition of the gas phase over the sample. The absorbed irradiation power for λ = 365 nm and λ = 385 nm is (10 ± 2) mW, P = 1.2 × 10−6 Torr.
A mixture of oxygen atoms containing the
16O and
18O isotopes can be fully characterized by three parameters:
P, α, and
Y, where
P represents the total pressure,
α is the percentage of 18O atoms among all O atoms in the gas phase, and
Y = 2α(1 − α) − C
34 represents the deviation of the mixture from the equilibrium with respect to the homo-molecular exchange reaction
16O
2 +
18O
2 ↔
16O
18O. Here C
32, C
34, and C
36 represent the current normalized concentrations of the molecules
16O
2,
16O
18O, and
18O
2 in the gas phase, respectively.
Y = 0 corresponds to the equilibrium mixture; if a mixture undergoing the homo-exchange has an initial non-zero
Y, it will tend to zero with time. The rate of photoactivated isotope equilibration in the gas phase (homo-exchange POIEq) is denoted as
Rq, expressed in molecules per second. In a closed volume at the pressures
P < 0.1 Torr, the POIEq process balances the mixture too quickly, thus we worked in a flow-through mode, with a continuous influx of fresh unbalanced mixture. This allowed us to continuously measure the rate of homo-exchange for an arbitrarily long time.
The hetero-exchange (POIEx) is the exchange between the isotope-enriched gas-phase molecules and the surface oxygen molecules or atoms (lattice or preadsorbed) of the sample (having a natural isotopic composition [
18O] = 0.25%). The rate of this process is denoted as
Rx and is expressed in atoms per second.
3.4. Modeling of the Kinetics of POIE O2 ⇄ ZnO/ZnO1−x/O−
The reflects all the stages of the process described in the article: photogeneration of an exciton → its diffusion to the surface → decay in the “dead zone” of a 2D surface structure into an electron and a hole → activation of F- and V-type centers by
e− and
h+ → interaction of activated centers with molecules of the gas phase → desorption of isotope-exchanged molecules into the gas phase.
We propose a four-channels model to describe the interaction between the O
2 gas and ZnO/ZnO
1−x/O
− during the POIE in a flow-through reactor: (1) O
2 photo-adsorption, (2) O
2 photo-desorption, (3) isotope exchange in the gas phase (homo-exchange), and (4) isotope exchange at the surface ZnO/ZnO
1−x/O
−– gas (hetero-exchange). The corresponding equations have been derived and the experimental data has been analyzed [
14].
The dependences of the rates of these processes were determined from the measured kinetic parameters of the outflow. The theoretical model was proposed to describe the decomposition of the interaction of O
2 gas with ZnO/ZnO
1−x/O
− through these channels.
. The radiative decay of an exciton is excluded by a 2D structure ZnO/ZnO1−x/O−, in which the exciton decays nonradiatively into a pair of long-lived (up to 8 × 103 s) electron and hole local states, on which a chemical reaction take place.
The equation for this process can be written in a vector-like form:
The notations in this equation follow:
The components of the vector $$\overrightarrow{N}$$ are the numbers of O
2 isotope molecules in the reactor with masses 32, 34 and 36. The module of this vector is defined as the sum of its components, $$N=|\vec{N}|=N_{32}+N_{34}+N_{36}$$. The α value is defined by $$\alpha=\frac{N_{36}+0.5\cdot N_{34}}{|\vec{N}|}$$ and means the fraction of the
18O isotope in the gas phase, while $$\overline{α} ≡ (1-α)$$ is the fraction of
16O.
Rx is the rate of homo-exchange, measured in [molec/s], and
Rq is the rate of hetero-exchange, measured in [atoms/s].
$$\vec F_{out}=\frac{\vec N}{\tau_{pump}}$$ is the outflow [molec/s], i.e., number of the molecules captured by MS in the time
τpump (reactor pumpdown time constant) and $$\vec{F}_{in}$$ is the inflow [molec/s] set in the experiment. The values $$\vec{N},R_x,R_q,\vec{F}_{out},\vec{F}_{in},R_{PA}$$ depend on time.
The first two terms on the right side of (1), $$\vec{F}_{in}-\vec{F}_{out}$$, correspond to the flow regime. The third term $$-R_{PA}\frac{\vec{N}}{|\vec{N}|}$$ is “photo-adsorption”. The fourth one $$\vec{R}_{PD}$$ is “photo-desorption”. The fifth one is “hetero-molecular exchange”, and the last one is “homo-molecular exchange”. The equation is a differential non-linear one, however the value under the differential is known, and the equation can be transformed into an algebraic one. In the experiments performed, photo-desorption was not observed, therefore, we consider it to be equal to zero.
The notation
allows to rewrite (1) as
In order to compare the theory to the experimental results we denote $$R_{x\_at}=\frac{A_{32}-A_{36}}{\alpha}$$ and $$R_{q}=\frac{A_{34}+R_{x_{-}at}\cdot\alpha\overline{\alpha}}{Y}-\frac{1}{2}R_{x_{-}at}$$.
The results obtained for
Rq and
Rx are shown in .
. Rates of kq = Rq/N and Rx over ZnO/ZnO1−x/O− under irradiation in the region of interband absorption at λ = 365 nm and exciton absorption at λmax = 385 nm. The incident photon fluxes were 1.8 × 1016 (±20%) quanta per second, P = 1.2 × 10−6 Torr. For POIE test reaction the irradiating in the exciton absorption band is about 5–8 times more effective than in the interband absorption.
Thus, the example of the POIE used as a model for redox reactions shows that the efficiency of the 2D structure ZnO/ZnO
1−x/O
− activated in the region of the resonant exciton excitation is ~5–8 times higher than in the region of the interband absorption [
13].
In summary, the prediction of A.N. Terenin is confirmed: the exciton discovered by E.F. Gross, being a neutral quasi-particle, can work in redox photocatalysis more efficiently than photo-generated charged particles: electrons and holes. For the first time, the 2D structure ZnO/ZnO1−x/O− was developed, in which the exciton energy is spent not on luminescence, but on the generation of long-lived (up to 8 × 103 s) F- and V-type centers that activate the photocatalytic redox reaction. The efficiency of the photocatalyst in the POIE reaction at the exciton excitation is ~5–8 times higher than that under interband absorption.
Thus, the 2D structure ZnO/ZnO1−x/O−, when activated in the region of exciton absorption, is expected to be a highly efficient photocatalyst.
This work was financially supported by RFBR under Grant 18-03-00754. The research was supported by “Nanocomposite”, “Physical Methods of Surface Investigation”, “X-ray Diffraction Centre”, and “Nanophotonics” centres of St. Petersburg State University. The authors are grateful to their colleagues: Titov, V.V., Tkalich, V.S., Mikhaylov, R.V., Akopyan, I.K., Labzowskaya, M.E. for performing experiments and discussing the results. B.V.N. acknowledges the support of St. Petersburg State University through the Grant ID 94033852.
Conceptualization; Methodology; Validation; Formal Analysis; Investigation; Resources; Writing-Original Draft Preparation; Supervision; Funding Acquisition.
The contribution of each of the co-authors for each item was 50%.
This research received no external funding.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.