Porous Carbon-Based Adsorbents for CO2 Sequestration from Flue Gases: Tuning Porosity, Surface Chemistry, and Metal Impregnation for Sustainable Capture
Received: 05 December 2025 Revised: 07 January 2026 Accepted: 13 January 2026 Published: 20 January 2026
© 2026 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
The issue of global warming is now widely understood to stem from the persistent release of large quantities of greenhouse gases (GHGs) by human activities, including carbon dioxide (CO2), chlorofluorocarbons (CFCs), methane (CH4), and nitrous oxide (N2O) [1,2]. Among all the GHGs, CO2 contributes dominantly to global warming [3]. Since the industrial revolution, the atmospheric CO2 concentration has risen dramatically from approximately 280 ppm to over 415 ppm, largely due to fossil fuel combustion and industrial processes [4]. Projections indicate it may reach about 450 ppm by 2035, with energy-related emissions rising to 43.2 billion metric tons, potentially leading to a 2 °C increase in global mean temperature [5]. This trajectory risks severe environmental and economic consequences, including disruptions to ocean chemistry [6]. Therefore, global efforts to mitigate CO2 emissions are critical, with targets aiming for significant reductions after 2030 and net-zero emissions between 2050 and 2060 [7].
More than 80% of the existing CO2 in the atmosphere results from power plants, industrial facilities and transportation [8]. Thus, capturing CO2 before its release into the atmosphere represents a crucial strategy for achieving decarbonization goals. To this end, various methods have been developed to capture CO2, which can be classified into three categories, including pre-combustion, post-combustion and oxy-fuel combustion [9,10]. Among these, post-combustion capture—which separates CO2 from flue gases after fuel combustion—is particularly amenable to integration with existing infrastructure, making it a widely adopted near-term solution [11,12,13].
To date, many technologies have been developed for CO2 capture and sequestration, including absorption, adsorption, membrane separation, micro-algal bio-fixation, and cryogenic fractionation [14,15]. As summarized in Table 1, each method presents distinct trade-offs in cost, selectivity, maturity, and environmental impact. Chemical absorption, for instance, is a mature technology but suffers from high energy penalties for solvent regeneration, equipment corrosion, and environmental concerns [16,17,18,19]. Membrane and cryogenic processes often face challenges related to cost, energy intensity, or effectiveness at low CO2 concentrations [20,21]. In this context, adsorption has gained considerable attention as a promising solid-phase capture technology. It operates based on the adhesion of CO2 molecules onto a solid adsorbent surface via physical or chemical interactions, offering advantages such as lower energy consumption, reduced corrosiveness, and easier regeneration [22,23,24].
Table 1. A comparison of post-combustion CO2 capture methods based on some key indexes.
|
Key Indexes |
Capital/Operational Cost |
Selectivity |
Recovery |
Reliability |
Maturity |
Environ. Friendliness |
|---|---|---|---|---|---|---|
|
Absorption |
High/high |
Low-moderate |
High (>90%) |
Low (corrosion, solvent losses) |
High |
No (absorbent emission) |
|
Adsorption |
Moderate/moderate |
High (specified adsorbent) |
High (>90%) |
High |
Moderate |
Very environmental- friendly |
|
Membrane |
High/moderate |
High |
Low-high (depending on the used membrane) |
Moderate |
Low |
Relying on the type of membranes |
|
Cryogenic |
High/high |
Low |
Low |
High |
Low |
Moderate |
|
Bio-fixation |
Low/high |
High |
Low |
Low |
Low |
Environmental-friendly |
The performance of adsorbents is largely governed by their textural properties (e.g., surface area, pore volume, pore size) and surface chemistry [25]. Consequently, porous materials with high surface areas and tunable pore structures are intensively investigated. Various adsorbents, including metal-organic frameworks (MOFs) [26], zeolites [27], oxide-based adsorbents [20], porous organic polymers (POPs) [28], porous polymer foams [29], and porous carbons derived from various precursors [8,16,30,31]. Numerous studies have been conducted primarily on the development of highly efficient adsorbents in terms of the adsorption capacity, energy efficiency/consumption, CO2 recovery, operating conditions and costs.
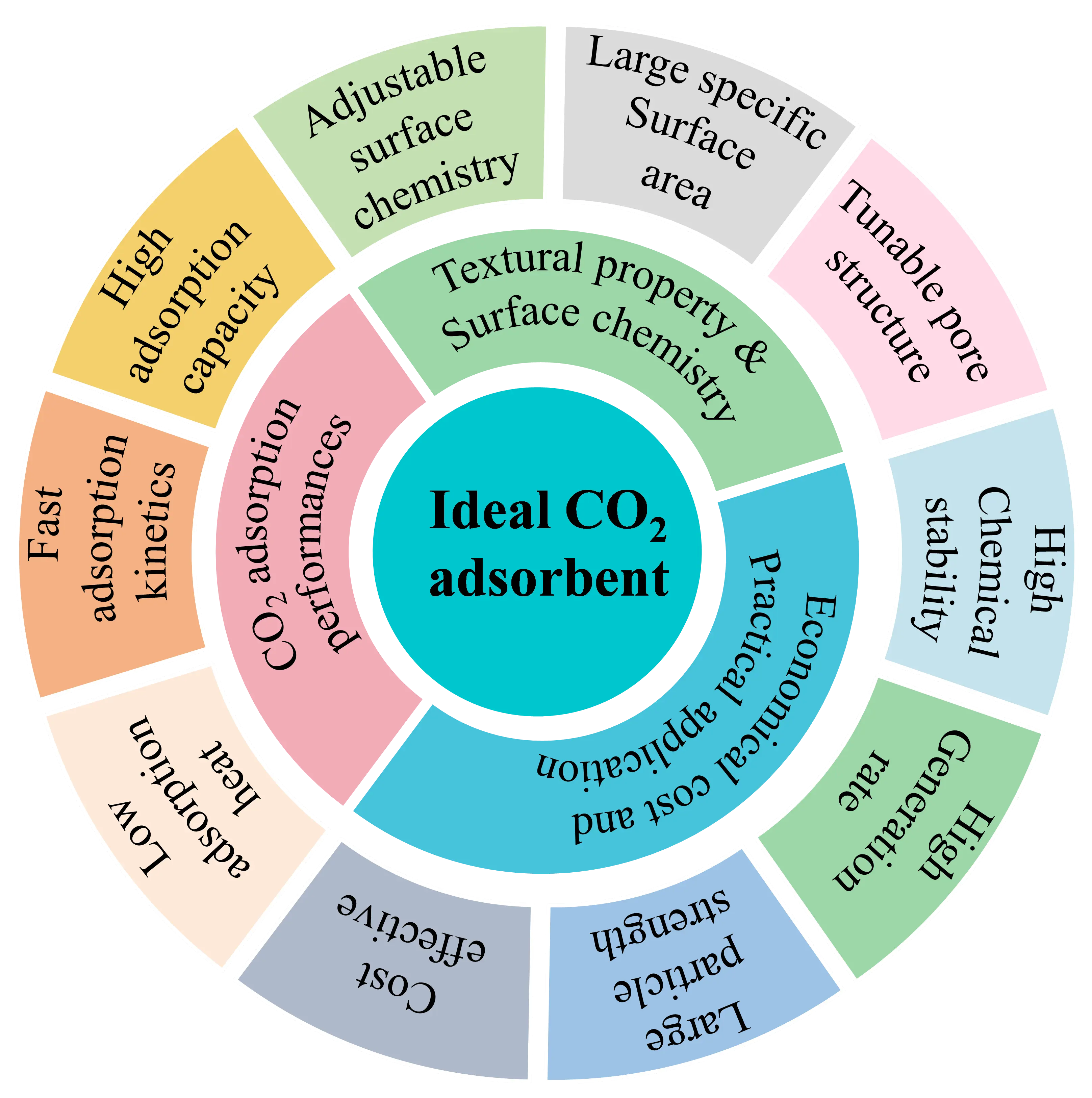
Among these solid adsorbents, porous carbons attracted the most attention as the adsorbents for CO2 capture [29], because they possess tunable pore structure and adjustable surface chemistry features that are suitable for CO2 adsorption at both low and high pressures [32]. Usually, the adsorption of CO2 by porous materials is mainly determined by the inherent properties of adsorbents, including the specific surface area (SSA), pore volume and size, and surface functional groups. Among these factors, the pore size is the key factor. It is recognized that porous materials rich in micropores or micro-mesopores are suitable for capturing CO2 at either low- or high-pressures [33,34], respectively. Of course, the adsorption performance will be affected by the operating conditions. Compared with other adsorbents, porous carbons (mainly embodied as activated carbons) possess almost all the desired characteristics of an ideal CO2 adsorbent, as illustrated in Figure 1. Furthermore, porous carbons have excellent water resistance, ensuring them a stable CO2 adsorption performance under high humidity flue gas conditions, so that they can meet the requirements of practical applications. Accordingly, porous carbons become the most popular and effective CO2 adsorbent [35].
A few reviews have been published focusing on the utilization of porous carbons for CO2 removal. These reviews provide a comprehensive overview of the existing porous carbons employed in CO2 capture, including the preparation of porous carbon materials, the summaries of adsorption performance and the effects of operating condition factors [10,28,29]. These reviews are mostly biased towards the narrative and conclusive; none of them focus on the strategies for enhancing the adsorption capability of CO2 capture in detail. For the researchers, understanding and mastering the strategies of how the modified porous carbons can enhance the CO2 adsorption performance will be beneficial to the development of effective porous carbons for CO2 capture. It is believed that a comprehensive and up-to-date review summarizing the recent strategies for enhancing the CO2 adsorption performance of porous carbons is necessary, which can not only serve as a useful starting point for getting into one of the most important fields, but also facilitate the intensive research on the rational and efficient design and development of more effective porous carbons for CO2 adsorption. Therefore, the present review aims to provide an overview of efforts to develop effective porous carbons for CO2 adsorption via various strategies. While significant progress has been made in enhancing the gravimetric adsorption capacity of powdered carbons under ideal conditions, their transition to industrial-scale post-combustion capture requires addressing broader challenges, including the evaluation under real flue gas environments, the formation of robust pellets, and the integration with scalable adsorption processes like fixed-bed or circulating fluidized-bed systems [36]. This review starts with a brief introduction to CO2 capture by adsorption. The second and third parts summarize the adsorption mechanism of CO2 combined with the impacts of operation conditions on the CO2 adsorption performance onto porous carbons. In the third section, the strategies to modify porous carbons for enhancing the CO2 adsorption performance were thoroughly reviewed. The limitations, challenges and future prospects in the applications of porous carbons for CO2 adsorption are addressed in the final section.
2. Mechanism of CO2 Adsorption by Porous Carbons
The exceptional CO2 adsorption performance of porous carbon materials (especially activated carbon) fundamentally stems from their unique disordered nanostructure. CO2, possessing a strong quadrupole moment but no dipole moment, interacts with the carbon surface through polar bonds at both ends of its linear structure [29]. As a classic representative of non-graphitized (or “hard”) carbon materials, activated carbon maintains a highly cross-linked microporous framework structure even under extreme temperatures (>2000 °C), thereby resisting graphitization. Modern structural studies utilizing advanced techniques, such as aberration-corrected transmission electron microscopy, reveal that this stability and its derived pore architecture originate from defect-rich sp2 carbon networks. Contemporary models propose that its structure is not merely a simple stacking of minuscule graphene monolayers, but also incorporates non-hexagonal carbon rings (such as pentagons and heptagons) embedded within the matrix. These topological defects introduce persistent curvature, forming a complex three-dimensional network composed of ultramicropores (often slit-like) and defect sites [37]. It is precisely this intricate nanostructure—characterized by high specific surface area, tunable pore geometry, and chemically active defect sites—that underpins the physical and chemical adsorption mechanisms of CO2 on porous carbon materials. The specific mechanisms have been thoroughly elucidated in relevant studies.
2.1. Physical Adsorption
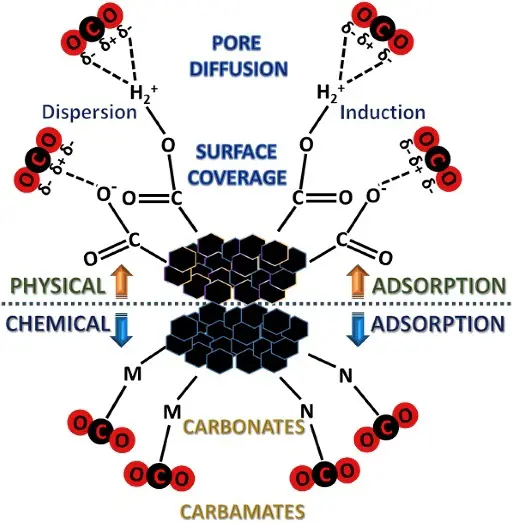
The adsorption mechanism of CO2 onto pristine porous carbon is illustrated in Figure 2. CO2 capture by porous carbon is primarily controlled by physical adsorption or physisorption. In the mechanism of physical adsorption, the CO2 molecules being adsorbed onto the surface of carbon sorbent mainly depends on the van der Waal’s attraction between the CO2 molecule and adsorbent surface, pole-ion and pole-pole interactions between the quadruple of CO2 and the ionic, and the polar sites of the solid adsorbent surface [33], which is verified from the reversible nature of the adsorption process. This adsorption process has a low heat ranging from −25 to −40 kJ/mole on carbon, which is comparable to the sublimation heat [34]. Even though the physisorption of the CO2 molecule on the carbon surface could be temperature-dependent as it approaches equilibrium, namely, lower temperature is beneficial to more CO2 being adsorbed, no activation energy is required for the occurrence of adsorption [38]. This is because of the linear shape and polar bonds on either end of CO2, which can readily interact with the active sites on the carbon surface. In addition, both dispersion and induction contribute to the attraction of CO2 to the carbon surface, correlating with the surface property [39]. Physisorption is recognized as an exothermic reaction with low enthalpy, owing to the weak Van der Waals attraction forces, and it is strongly dependent on the textural properties of the carbon material, such as specific surface area and micropore volume [40], while it is linked with adsorption energy. Beyond the classical Van der Waals forces, the intrinsic molecular properties of CO2 play a pivotal role in physisorption within carbon frameworks. CO2 possesses a significant quadrupole moment, which induces strong electrostatic interactions with the local electric fields generated by surface functional groups or structural defects. In ultramicropores (<0.8 nm), the overlapping potential walls from opposing pore surfaces significantly amplify these quadrupole-field interactions. This explains the enhanced CO2 uptake at low partial pressures, as the energetic affinity of the pore system is dictated not only by pore volume but also by the electrostatic environment of the carbon matrix.
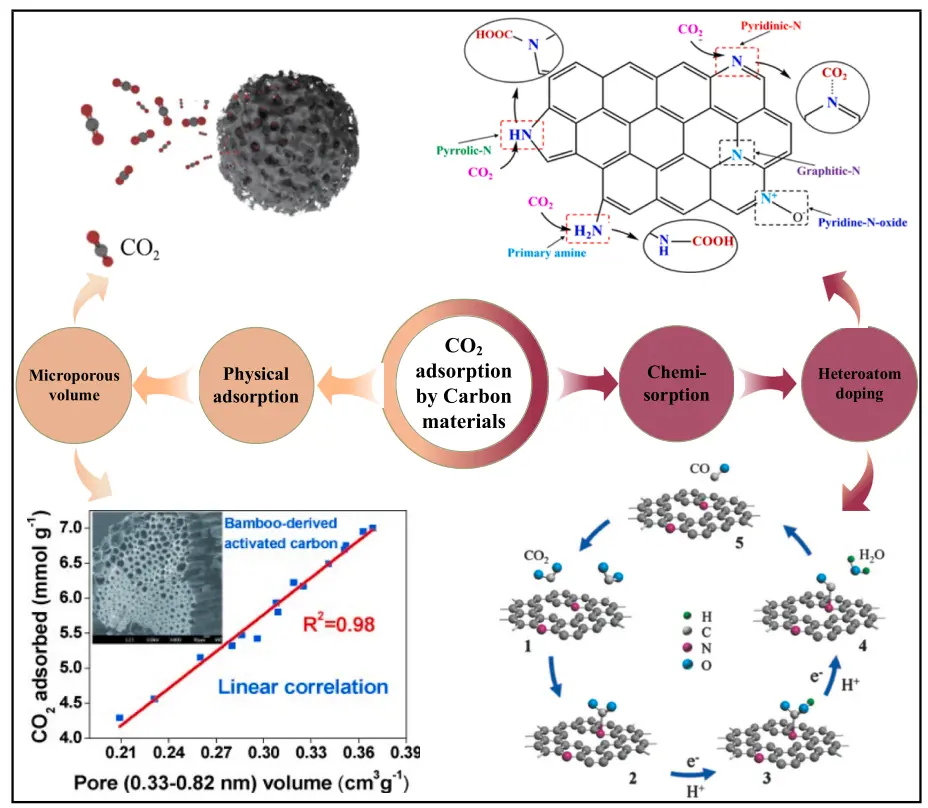
The adsorption performance of physisorption predominantly depends on the textural properties of carbon, facilitated by the intermolecular forces between activated carbon and CO2. Porous carbon with a well-developed pore structure is crucial for CO2 adsorption, and the primary texture parameters include specific surface area, pore volume, pore size, and its distribution, which determine the adsorption capacity of CO2. Recent studies further emphasize the importance of hierarchical porous architectures derived from biomass waste for high-performance CO2 capture [41]. Dai et al. [42] prepared rice husk derived porous carbon using KOH activation, having a specific surface area of 1496 m2/g, total pore and micropore volume of 0.786 cm3/g and 0.447 cm3/g. This porous carbon exhibited CO2 adsorption capacity of 5.83 mmol/g at 273 K and 1 bar and good CO2/N2 selectivity of 9.5. Deng et al. [43] fabricated bamboo derived porous carbon by KOH corrosion activation, which was rich in a lot of ordered micropores. The study disclosed that a perfect linear relationship existed between the pore size and CO2 adsorption capacity, and the narrow micropores with a diameter of 0.55 nm were identified as the primary pores for CO2 adsorption, exhibiting an adsorption capacity of 7.0 mmol/g. Silvestre-Albero et al. [44] investigated CO2 adsorption onto carbon molecular sieves, and confirmed that CO2 adsorption is not only related to the specific surface area, but is also strongly correlated with the micropore volume. Particularly, the developed, uniform and narrow micropores are the key to CO2 adsorption. The significance of micropores of carbon materials in CO2 adsorption was also validated by other studies [45,46,47,48].
Besides the textural properties, the chemical nature of carbon materials also plays a key role in CO2 adsorption. The chemical nature can be defined by the heteroatoms present in their matrix, such as nitrogen, hydrogen, oxygen, phosphorus, and sulfur. These heteroatoms can originate either from the inherent compositions of the carbon precursor or the activation process introduced by the activating agent [50]. Specifically, the adsorption capacity and selectivity towards CO2 depend on the surface functional groups formed by these heteroatoms and the delocalized electrons of the carbon structure. Detailed experimental and DFT studies have shown that the CO2 adsorption capacity of N-doped carbons is governed by a threshold effect: at high nitrogen content, pyridinic-N (N-6) enhances localized polarity, whereas at low doping levels, pyrrolic-N (N-5) improves adsorption through conjugation effects [51]. Furthermore, studies combining experimental data with DFT calculations have recently unveiled the critical role of specific topological defects; for example, pentagonal topological defects were shown to work synergistically with N/O co-doping to enhance CO2 adsorption by regulating the electronic distribution and promoting electron transfer on the biochar surface [52]. Among these heteroatoms, nitrogen (N) is the most popular heteroatom that is incorporated into the carbon matrix to improve the adsorption capacity and selectivity of CO2 adsorption [29,53]. Recent DFT studies on Fe-N co-doped biochar have further demonstrated that the synergy between Fe and N generates Fe-N active sites, which enhance surface alkalinity and significantly boost CO2 capture [54]. The introduction of nitrogenous groups like amide, nitrile, amines and others can increase the physisorption of CO2 on carbon surface [29,48], due to the strong interaction between the acidic CO2 and basic nitrogenous surface functional groups. The basic character of carbon is closely related to the resonation of π electrons present in carbon aromatic rings, which attract protons and nitrogen-containing groups. On the contrary, the acidic character of carbon is tightly linked with the presence of oxygen-containing groups, and the acidity will be greater when the oxygen concentration on the carbon surface increases.
2.2. Chemical Adsorption
In chemical adsorption of chemisorption, an acidic CO2 molecule is attached to the active site on the carbon surface via covalent bonding. The chemisorption of CO2 onto adsorbents can be evaluated by characterizing the used carbon after adsorption and by identifying the heat of adsorption. During the adsorption process, the CO2 gas undergoes a covalent chemical reaction to connect with the specific active sites on carbon, with a substantially high adsorption heat that is almost equal to the heat of reaction [34]. Compared with physisorption, chemisorption has a much higher heat of adsorption, ranging from 40–400 kJ/mol [55], owing to its strong chemical bond. Moreover, CO2 is adsorbed on the carbon surface and chemically bonded to form carbonates. Generally, CO2 chemisorption is irreversible. The formed carbonates usually include bicarbonate, bidentate carbonate, and monodentate carbonate, which correspond to weak, medium, and strong adsorption basic sites [47].
Chemical adsorption of CO2 by porous carbon is generally accomplished through doping various heteroatoms onto porous carbons. The most frequently adopted methods include doping nitrogen, comprising urea, melamine, p-Phenylenediamine, polyethyleneimine, polyaniline, etc. Nitrogen-containing functional groups can lead to chemisorption via the formation of a zwitterion or a carbamate, and the hydration of CO2 to form bicarbonate [38]. Tan et al. [39] investigated the adsorption of CO2 on nitrogen-doped porous carbon prepared from licorice residue and urea, revealing a synergistic effect between the nitrogen content and the activated carbon’s structure on CO2 adsorption performance. They found that nitrogen content contributed remarkably to CO2 adsorption, attributed to the acid-base reaction between CO2 and doped nitrogen atoms. CO2 is recognized as a weak Lewis acidic gas that can react with doped nitrogen-containing groups that act as Lewis bases due to their negatively charged and electron-donating behavior [42]. The chemical interaction between N-containing functional groups and CO2 involves more than simple acid-base neutralization. For amine-functionalized or N-doped carbons, the reaction typically follows a zwitterion mechanism. In the absence of moisture, a CO2 molecule undergoes nucleophilic attack by the lone pair of electrons on the nitrogen atom to form a zwitterion intermediate, which subsequently undergoes proton transfer to form a stable carbamate: 2RNH2 + CO2 ↔ RNHCOO− + RNH3+. Under humid conditions, the mechanism shifts towards a bicarbonate pathway, where water acts as a proton mediator, theoretically doubling the stoichiometric capture capacity per nitrogen site: RNH2 + CO2 + H2O ↔ RNH3+ + HCO3−. In addition, Wang et al. [44] examined the adsorption performance of CO2 on a series of nitrogen-doped porous carbon, and an enhanced CO2 adsorption capacity was observed. The nitrogen species, such as pyridine-N, pyrrole-/pyridine-N, graphitic-N, pyridine-N-oxide and primary/secondary amines, can react with CO2, forming graphitic-N, carboxyl groups and heterocyclic structures (pyrrolic-N), thus facilitating the CO2 adsorption. This is corroborated by a recent study on N, S co-doped porous carbon derived from coconut shell, where the presence of pyridinic-N and pyrrolic-N significantly contributed to a high CO2 uptake of 4.38 mmol/g at 25 °C, demonstrating the critical role of these N-functionalities in enhancing surface basicity and Lewis acid-base interactions with CO2 [56]. In addition to nitrogen, other atoms, for example, oxygen, sulfur, boron, phosphorus, etc., can be doped and facilitate the chemical adsorption of CO2 on heteroatom-doped porous carbon materials.
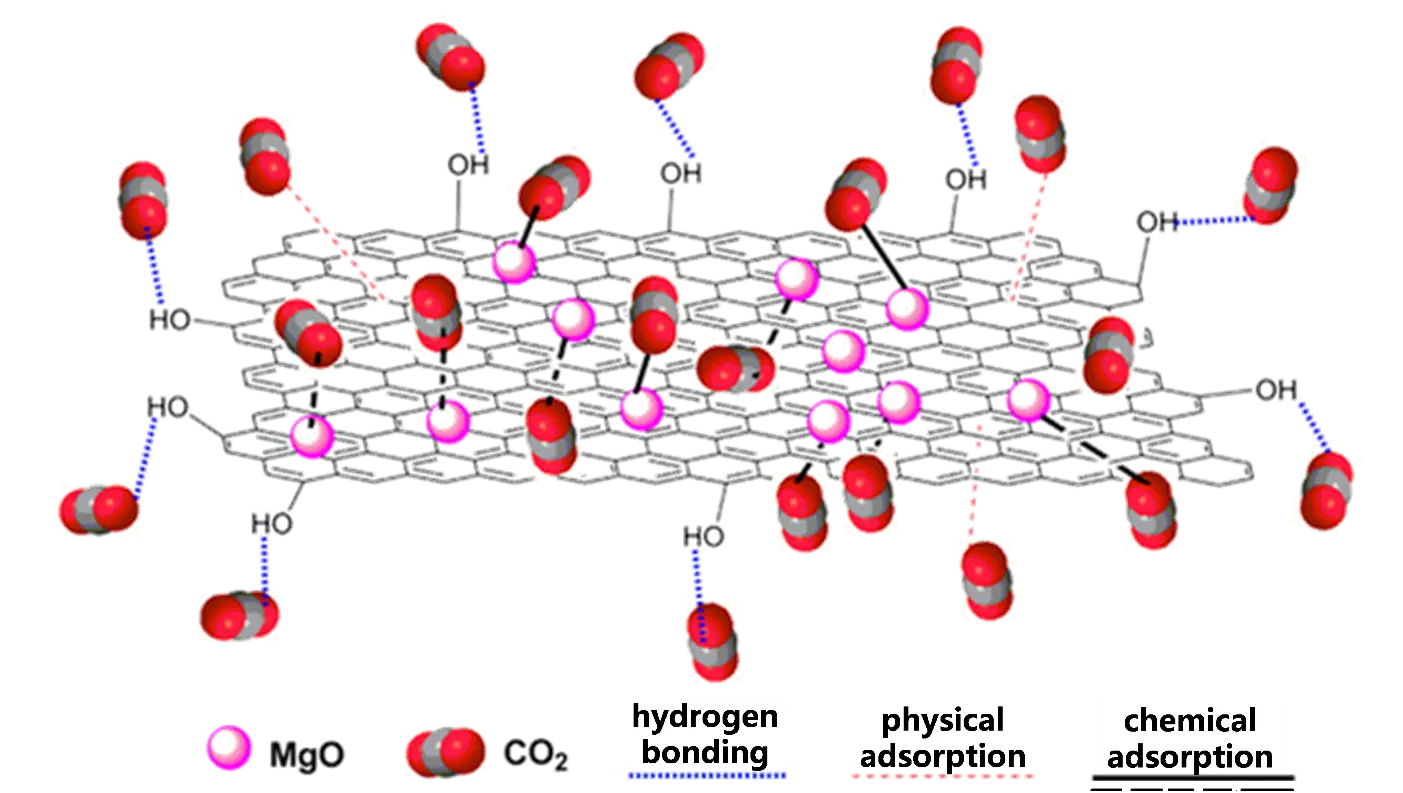
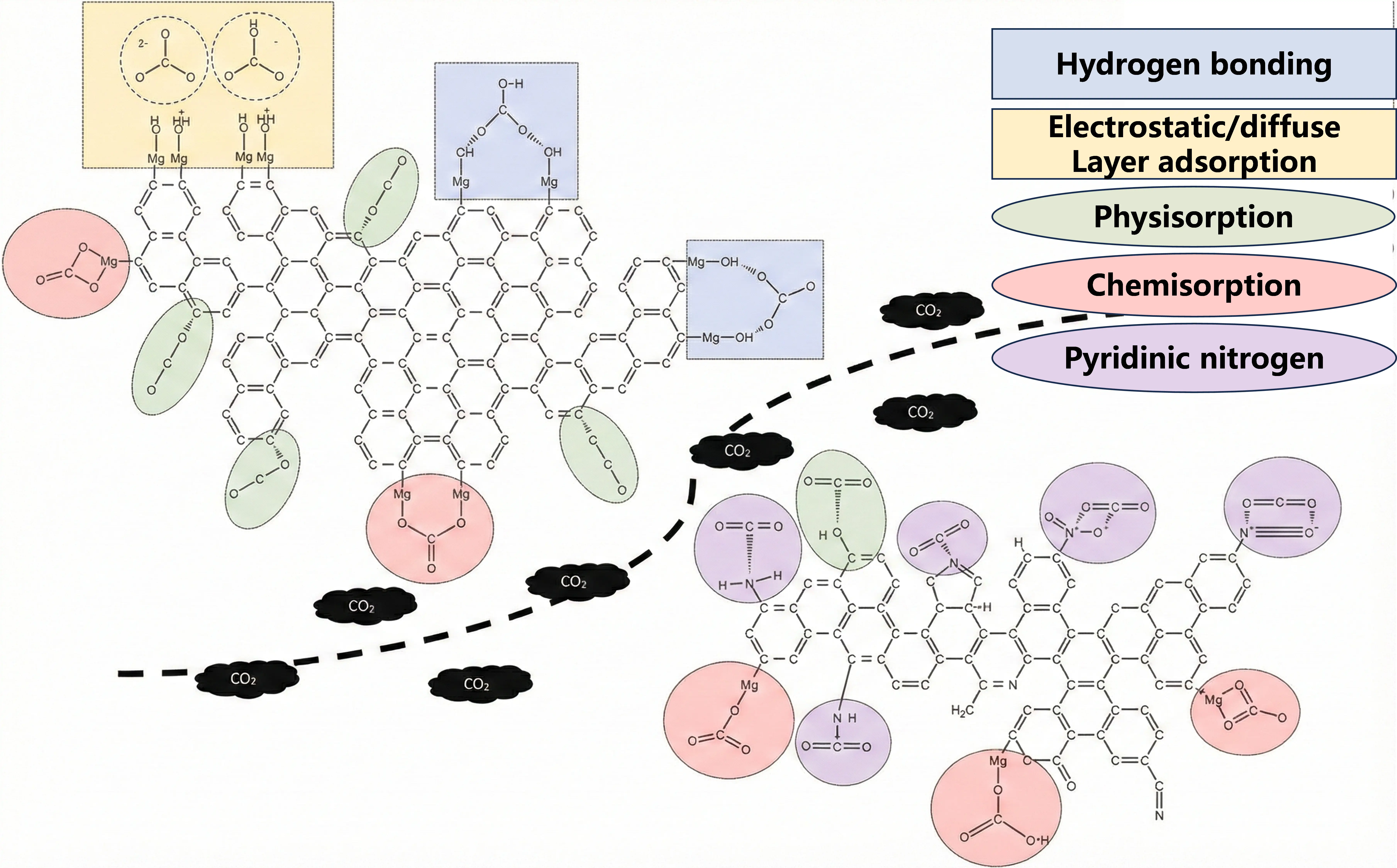
Apart from heteroatom doping, metal impregnation on carbon has been demonstrated to have excellent stability and prominent adsorption enhancement, especially via chemisorption. Numerous studies have clearly proved that the impregnation of metal ion into biomass derived porous carbon can integrate their multiple advantages effectively, leading to a much-enhanced CO2 adsorption performance [45,46,47]. The presence of metal ions will result in the formation of bidentate and monodentate carbonates owing to the bonding between CO2 and metal ions (Mg2+) and/or O2− [48]. In addition, the strength of basic sites of carbons is proportional to the amount of coordinated electronegative metal ions, which can be boosted by employing metal-impregnated carbon with a high specific surface area, providing more space for O2− sites [50]. More importantly, the impregnation of metal ions can greatly improve the adsorption capacity of CO2 under humidity. Generally speaking, the presence of H2O will significantly inhibit the adsorption of CO2 onto carbon [57]. While for metal-impregnated carbons, for example, MgO-based and Mg(OH)2-based carbons are capable of generating MgCO3 in the presence of H2O [58], thereby improving the CO2 adsorption capacity. The water vapor will serve as a channel for enhanced chemisorption interactions between MgO and CO2, producing CO32− and H+ ions when water surrounds MgO. Besides magnesium ions, other metal ions can be impregnated into carbon to enhance the chemisorption of CO2 onto carbon, which will be discussed in detail later.
The adsorption mechanism of CO2 onto carbon materials can be summarized in Figure 3, involving both the physical adsorption promoted by the porous structures and chemisorption induced by doping heteroatoms or impregnating metal ions. Carbon materials will continue to undergo extensive investigation in CO2 adsorption; some challenges, including low adsorption efficiency, high operation expenses and weak moisture/impurities tolerance, should be addressed. Hence, elucidating the adsorption mechanism is very crucial to the theoretical exploration and industrial applicability of carbon materials for CO2 capture.
3. Impacts of Operation Conditions on CO2 Adsorption
The CO2 capture in actual applications is carried out in various kinds of complicated conditions, and the operating parameters will inevitably influence both the capacity/extent and rate of CO2 adsorption. The typical operating parameters include temperature, partial pressure of CO2, water vapor, and impure gases (NOx, SOx, H2S, etc.). It is necessary to review and clarify the impacts of CO2 adsorption on porous carbons, for developing appropriate adsorbents that can effectively work under these conditions and still have high adsorption capacity.
3.1. Adsorption Temperature
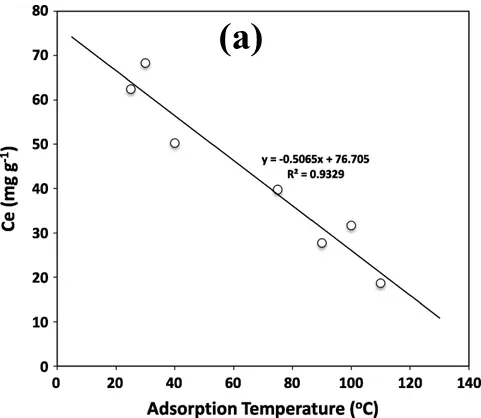
The adsorption temperature exercises an important influence over the adsorption performance from both physical and chemical adsorption [59,60]. The variation of temperature itself possesses a propensity for either negative and positive (sometimes conflicting) effects on individual aspects of CO2 adsorption. On one hand, adsorption is exothermic, so it is predicted that the CO2 adsorption capability increases as the adsorption temperature decreases [61]. On the other hand, CO2 molecules have high kinetic energy at high temperatures, so they can move faster, resulting in less adsorption time on the adsorbent surface, which leads to reduced adsorption capacity. Although the increased energy enables a greater diffusion rate of gaseous molecules, the possibility of CO2 being adsorbed or retained on the carbon surface by fixed energy adsorption sites is decreased. As shown in Figure 4a, the CO2 adsorption capability of sugarcane bagasse derived carbon decreased linearly (R2 = 0.93) with the increase of temperature, which confirms a strong negative correlation between CO2 adsorption capacity and adsorption temperature.
3.2. Adsorption Pressure
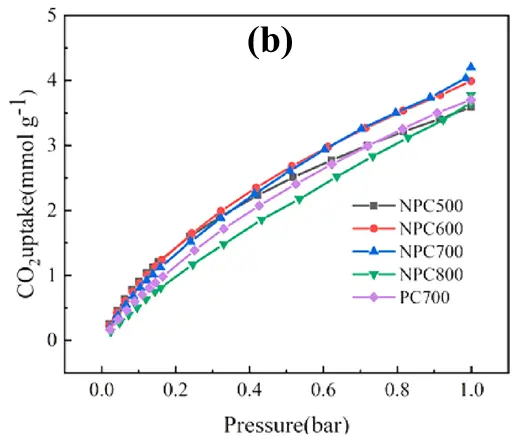
There is a considerable demand for more studies on the adsorption capacities of carbon materials at higher pressures. Numerous researches have proved that the operating pressure plays a significant effect, and CO2 adsorption capacity increases with the elevation of CO2 pressure [63,64,65]. As shown in Figure 4b, the porous carbon and nitrogen-doped porous carbon all exhibited enhanced CO2 adsorption capacity with the elevation of pressure ranging from 0 to 1.0 bar, and the maximal CO2 adsorption capacities of 3.59–4.20 mmol/g were obtained at 25 °C and 1.0 bar [62]. When a gas molecule is adsorbed onto a solid surface, the global volume of the adsorbent will decrease. According to Le Chatelier’s principle, the equilibrium state will shift toward a new state when a stress (for example, a change in pressure) is applied on a system, and the new state will counteract the applied stress. To counter the increase in pressure, the number of gas molecules in the system tends to be reduced, thereby the adsorption capacity of CO2 on the adsorbent will increase [66,67].
Furthermore, the investigation on the adsorption capacity at varied pressures can gain insights into the role of each materials property of porous carbons on CO2 capture capacity. The previous studies demonstrated that CO2 capture capacity at low pressure of 0.1–0.2 bar was more correlated with the practical post-combustion application [47,55], which was consistent with the partial pressure of CO2 in flue gas. In fact, the CO2 capacity on porous carbon is determined synergistically by the adsorbent properties (such as textural properties and chemical compositions) and the operating conditions, while their impacts are not independent. To determine the underlying dependent relationships between CO2 adsorption capacity and adsorbent properties and operating conditions, machine learning (ML) methods were recently adopted, which can identify complex nonlinear relationships among multiple influencing factors and interdependent variables. Shang et al. [59] developed robust ML models to predict the CO2 adsorption capacity of porous carbon based on the adsorbent properties and adsorption conditions. They found that pressure played a dominant role in determining CO2 adsorption capacity at low pressure (0–0.2 bar), while the relative contribution of pressure declined with increasing pressure. In addition, the textural properties are more vital to CO2 adsorption than chemical composition across different pressure and temperature ranges; the relative importance of ultra-micropores increased with increasing pressure.
3.3. Water Vapor
In actual CO2 capture, water is one of the most frequent impurities in the gas stream, especially, water vapor is ubiquitous in the flue gas and in lots of other CO2-rich industrial gases. H2O is usually the third most abundant component in flue gases after N2 and CO2, followed by O2. The water content in flue gases is 6–18% depending on the origin [60], while it is typically 6–7% in biogas [61]. H2O can be strongly adsorbed by many inorganic adsorbents, for it is a polar molecule, so the presence of H2O in the mixture can cause some problems for adsorbents. For example, competitive adsorption will occur between CO2 and H2O, and the adsorption capacity for CO2 will be weakened for the adsorbents that have a higher affinity toward water molecules than CO2 molecules [68,69]. For this reason, a dehumidifying unit was proposed for H2O removal from the feed gas prior to entering the capture unit, such as inserting a layer of a moisture adsorbent material (e.g., alumina, alumina-zeolite) [70,71]. Nevertheless, the dehumidification units will increase the overall capital and operating costs [72,73]. Although carbon adsorbents are hydrophobic, showing fewer issues with water vapor, it is still required to study the humidity effect on carbon adsorbents for CO2 adsorption, given the ubiquitous nature of water in gas streams.
Porous carbons generally exhibit type-I adsorption isotherm for CO2, indicative of their high affinity toward CO2 at low partial pressures [74]. Moreover, they often show low affinity toward water vapor at low relative humidity (RH) with low adsorption capacity, while the water uptake increases dramatically at higher RH levels, which is reflected as the “S”-shape adsorptions corresponding to type-V isotherm [75,76]. It is recognized that water adsorbs first over surface functional groups due to its strong affinity for water, then on top of the chemisorbed water molecules via hydrogen bonding, based on the model proposed by Do and Do [77]. Eventually, water clusters will form on the surface of porous carbons and move into their micropores, as established earlier based on experimental and theoretical results [78,79,80]. Subsequently, the generated water clusters contributed to micropore filling within the adsorbent, potentially occupying the micropores completely, leading to competitive adsorption between the water and the target gas (CO2).
Most investigations on carbon-based adsorbents carried out under subatmospheric pressures reported negative impacts of humidity on CO2 uptake, as a result of the competitive adsorption with water. Duran et al. employed one commercial carbon (Norit R) and two biomass-derived porous carbons for CO2 adsorption from a flue gas stream, and single-component adsorption isotherms of N2, CO2 and H2O were measured to evaluate the selectivity of CO2 to water and nitrogen at 30–70 °C [81]. They found that water was adsorbed preferentially on all the carbon samples compared with CO2, especially at the lowest temperature. When 2 vol% H2O/N2 and 2 vol% H2O/8 vol% CO2/N2 gas mixtures corresponding to 48 and 16% RH at 30 and 50 °C was used, water uptake was hardly affected by CO2. On the contrary, the presence of water reduced CO2 uptake remarkably at the highest RH, and the prehydration of the adsorbents at 30 °C reduced CO2 uptake by as much as 46%. For the biomass-derived porous carbons, their surface functionality is a determining factor for water adsorption in terms of hydrophobicity and hydrophilicity. How the surface hydrophobicity of biochar varies with the feedstock and preparing conditions is controversial based on the reported studies. Some proposed that the hydrophobicity changed with the activation method [82] and the increase of production temperature [83,84,85]. The details are discussed as follows.
Younas et al. [82] investigated the influence of activation method on the competitive adsorption of water vapor and CO2 onto a palm shell derived porous carbon, and both physical activation and chemical activation using 20–50 wt% NaOH solutions were used to prepare porous carbons. They found that the presence of water vapor (20% RH) can reduce CO2 uptake, and the negative effect of humidity was more severe for physically than chemically activated porous carbons, with 45% and 13% declines, respectively. Gray et al. [83] inspected the influences of porosity and hydrophobicity of biochar on water uptake. The biochar was produced from two feedstocks (hazelnut shells and Douglas fir chips) at three production temperatures (370, 500 and 620 °C). The production temperature changed the surface hydrophobicity, and the hydrophobicity decreased with an increase in the production temperature, which can be ascribed to the volatilization and loss of the aliphatic functionality at higher production temperatures. Contrary to the studies by Gray et al., some other works reported opposite results, where the hydrophobicity increased with increasing production temperature. These studies assessed hydrophobicity by measuring atomic ratios such as O/C, H/C and (O+N)/C [86,87] raising the pyrolysis temperature would result in the reduction of both ratios [88], leading to a subsequent increase in hydrophobicity due to the loss of the polar functional groups.
At high pressure, there is an extensive consensus regarding the beneficial effect of water on CO2 adsorption onto carbon adsorbent. Zhou et al. [89] measured CO2 adsorption isotherms of coconut shell derived microporous carbon at 275 K up to 40 bar over prehydrated adsorbents. The moisture content Rw, was defined as the ratio of adsorbed water to carbon. Figure 5a revealed that the moisturized samples had lower CO2 uptake than the dry carbon at low pressure, and their CO2 uptake increased significantly at 15 (20 bar), while the CO2 uptake on dry carbon was almost stable over 15 bar. This was ascribed to the enhanced solubility of CO2 in water at high pressure, resulting in the formation of CO2 hydrate. Figure 5b demonstrates that the inflection point occurs at higher pressure as temperature increases, and the point values are comparable to the pressure required for the formation of CO2 hydrate in pure water. Unlike that on the exclusively microporous carbons, the CO2 adsorption isotherms over micro-/mesoporous carbons showed similar transitions in the presence of water, but no plateau was observed with the formation of CO2 hydrate [29].
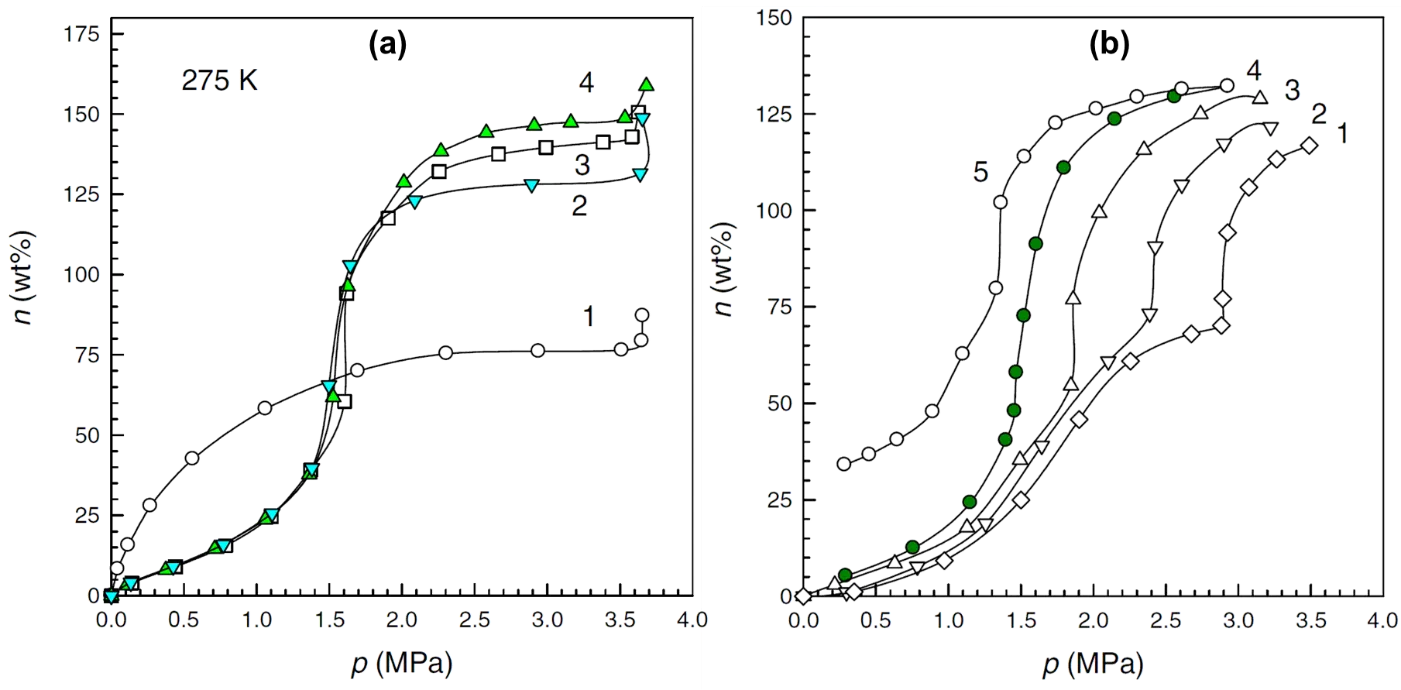
Figure 5. CO2 adsorption isotherms on a microporous carbon (a) with different moisture contents at 275 K. 1: Rw = 0, 2: Rw = 1.74, 3: Rw = 1.32 and 4: Rw = 1.65; (b) different temperatures under Rw = 1.31. 1: 281 K; 2: 279 K; 3: 277 K; 4: 275 K (adsorption); 5: 275 K (desorption). [Reproduced with permission from ref. [89]. Copyright 2007 Elsevier].
3.4. NOx, SOx, H2S, CH4
Numerous studies available have focused on the adsorption of pure CO2 on carbon adsorbents [90,91,92], while CO2 is hardly produced as a pure component. In addition to the mentioned water vapor, there are other gas impurities, including NOx, SOx, H2S and CH4, which also can influence the adsorption efficiency of CO2 onto carbon adsorbents [93]. Usually, NOx and SOx in the gas stream will react with or be adsorbed on the adsorption sites, forming complexes with adsorbed compounds, leading to a decrease in the number of sites available for the adsorption of the CO2 molecule. Accordingly, clarifying the impacts of these impurities on CO2 adsorption is essential to determine the feasibility of an effective adsorbent [94].
NOx is a byproduct produced during fuel combustion, which can be adsorbed by porous carbons via primarily chemical process, depending on the presence of oxygen in the flue gas instead of the structural properties of the adsorbents. Zhang et al. [95] investigated the NO adsorption onto different porous carbons with various pore structures and surface functional groups. They found that virtually no NO can be removed in the absence of oxygen, while a large amount of NO was removed rapidly through adsorption and oxidation facilitated by porous carbons that acted as both adsorbent and catalyst. The NOx removal can be enhanced on the metal-loaded and heteroatom- doped porous carbons. Chen et al. [96] studied NO adsorption on ordered mesoporous carbon (OMC) and cerium loaded OMC, finding that oxygen played the key role in NO adsorption, and physical adsorption was the main mechanism of NO removal without oxygen. Additionally, the OMC displayed twice the NO adsorption capacity as the disordered porous carbon, and the introduction of cerium into the OMC enhanced the NO adsorption.
The adsorption of SOx on carbon materials is principally dependent on physical adsorption facilitated by the porous structure and chemical adsorption by surface alkaline functional groups on carbon. Sun et al. [97] inspected the adsorption of SO2 on activated carbon and carbon nanotube, finding that SO2 was mainly adsorbed in the micropores, since the micropores contained strong potential energy fields enabling SO2 to be easily adsorbed onto carbon. A wide pore size distribution is not beneficial to the generation of adsorption potential energy fields, the SO2 adsorption will be reduced. Sethupathi et al. [98] examined the adsorption performances of CO2, H2S and CH4 by four different biomass derived porous carbons produced from perilla leaf, soybean stover, Korean oak and Japanese oak, and the adsorption tests were conducted in a continuous fixed bed. The porous carbons exhibited negligible adsorption of CH4 even without any of the other gases, which was attributed to the large pore size (>1 nm) allowing CH4 molecules to slip. All the carbons exhibited good adsorption performance for pure CO2 and H2S, while the adsorption capacities toward CO2 decreased by 90–95% when the gas mixture was employed. This was because of the competitive adsorption between CO2 and H2S, where H2S was preferred to be adsorbed. It can be concluded that the acidic gases such as H2S can inhibit CO2 adsorption on carbon surface.
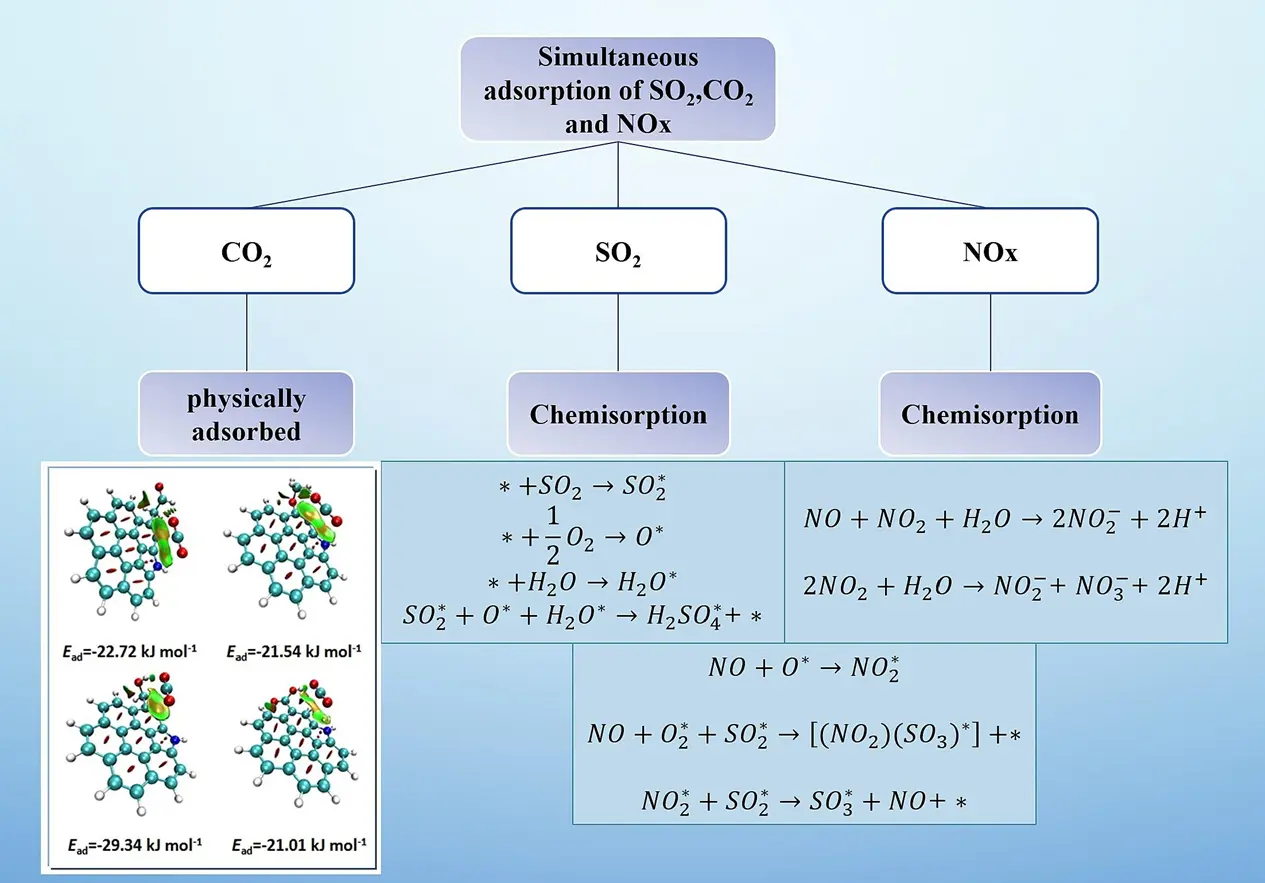
Great progress has been achieved in dealing separately with the three common acidic gases found in flue gas, namely CO2, SO2 and NOx, while there is less research on simultaneously removing these three acidic gases through adsorption. On the other hand, it is crucial to understand the mutual influence among the three gases in the adsorption process to improve the adsorption capacity, especially for capturing CO2 in the presence of other acidic gases. Zhou et al. [63] investigated the co-adsorption behaviors of CO2, SO2 and NO that exist in flue gas onto porous carbon by experimental measurement and theoretical model fitting. They found that the Freundlich model was more accurate than the Langmuir model in predicting the adsorption amount, the adsorption affinity of porous carbons towards the three gases followed the order: SO2 > CO2 > NO, and the sequence of required work was as follows: CO2 > NO > SO2. These findings provide some theoretical foundation for removing CO2 by adsorption onto carbon adsorbent in the presence of other acidic gases.
Yi et al. [64,65] examined the simultaneous adsorption of CO2, SO2 and NO on metal-loaded porous carbons. It was found that the Cu-loaded carbon showed better adsorption performance than those porous carbons loaded with other metals such as Ca, Mg and Zn, and the adsorption capacities were 0.367, 0.357 and 0.090 mmol/g for CO2, SO2 and NO, respectively. Furthermore, they found that high temperature impacted negatively the adsorption of these gases and low temperature favored their adsorption on porous carbon. The adsorption capacity of SO2 and NO can be improved in the presence of oxygen, and the oxidation of them into SO3 and NO2 would be promoted by oxygen. Moderate amounts of water vapor can enhance the adsorption capacity of SO2, while reducing the adsorption of CO2 and NO owing to their low solubility in water. Finally, the adsorption mechanisms of CO2, SO2 and NO are illustrated in Figure 6. The acidic gases, including CO2, SO2 and NO, can be adsorbed simultaneously over carbon materials. However, the main adsorption mechanism toward them varied to some extent. Among them, CO2 is primarily adsorbed by physical adsorption, while SO2 and NO are adsorbed mainly by chemical adsorption. Up to now, limited research has been conducted on the simultaneous adsorption of them. Therefore, overcoming the mutual influence of these gases during the adsorption process remains both necessary and challenging. Initial insights into such complex interactions come from studies on binary gas mixtures relevant to flue gas compositions. For instance, research on the co-adsorption of CO2 and acetone (a model VOC) on N-doped biochar revealed an initial competitive adsorption followed by a synergistic phase where acetone enhanced CO2 uptake, likely through dissolution and capillary condensation effects, while CO2 itself inhibited acetone adsorption [99]. It is expected to achieve satisfactory removal results by selecting suitable loading and doping functional groups on carbon materials.
4. Strategies for Enhancing CO2 Adsorption on Porous Carbons
4.1. Tuning Porosity
The textural properties of porous carbon adsorbents are the dominant factors that determine their adsorption performance towards CO2 capture. The pores in porous carbons can be classified into micropore (pore size/width (d) < 2 nm), macropore (d > 50 nm) and mesopore (2 nm < d < 50 nm), relying on the size of the pore. It is generally recognized that the adsorption of small molecules like CO2 is principally dependent on the micropore filling mechanism, and the CO2 adsorption capability of porous carbon is mainly correlated with the narrow microporous structure [102,103]. Previous studies have verified that micropores with a size less than 1 nm, especially ultra-micropores (d < 0.8 nm), are much more beneficial to CO2 uptake at ambient pressure [104,105], and they can improve the selectivity of CO2 to N2 [106]. As an ideal adsorbent, additional properties besides the adsorption capacity, i.e., good regeneration ability, rapid adsorption kinetics, stability, etc., are key to the adsorbents in practical applications [107,108]. Although the mesopore and macropore in porous carbons contribute a little to the adsorption capacity of CO2, they supply channels for rapid diffusion of CO2 and are beneficial to the desorption; then the porous carbon materials in the absence of mesopores usually have poor regeneration ability [109]. In this regard, continuous efforts have been devoted to tuning the pore structure of carbon adsorbents with appropriate hierarchical pore size and pore size distribution for improving their CO2 adsorption performance.
How to fabricate porous carbon with a suitable pore structure has been the focus of research in the CO2 adsorption by porous adsorbents. The fabrication of porous carbon generally requires two steps: carbonization, in which the raw materials are graphitized and activation for tuning the porosity. The overall number of pores, the shape and size of pores, as well as the pore size distribution, determine the adsorption capacity, as well as the adsorption rate. The carbon materials by merely carbonizing the raw materials usually have little pores, which are averse to CO2 adsorption. Some carbonized products have higher porosity, but their pore size distributions are not ideal, in which the pore diameter/width is bigger than the pore size required for CO2 separation with poor selectivity, on account of the heterogeneous composition and structure of the carbon-containing raw materials. To render the as-fabricated porous carbons show better adsorption performance for CO2, it is vital to further tune the pore structure after carbonization by various activation methods. Activation control is commonly used to tune the pore size of porous carbon, which can make the pore structure much more developed by opening or expanding the pores.
Chemical activation by using KOH, K2CO3, ZnCl2, etc., as activating agents is one of the most effective pore creation and regulation methods. Beyond traditional chemical activators, innovative media such as molten salts have been employed to achieve uniform and deep micropore development. For instance, a one-step molten salt thermal treatment using a NaOH-Na2CO3 system was shown to effectively integrate carbonization and activation, producing N/O co-doped biochar with a high specific surface area (up to 3312.89 m2·g−1) and a well-developed microporous network, leading to a CO2 uptake of 6.02 mmol·g−1 at 273 K and 1 bar [110]. Deng et al. [111] fabricated porous carbons derived from peanut shells and sunflower seed shells using KOH as an activating agent, and the optimal porous carbons were achieved at a low KOH/C ratio of about 1.0. The porous carbons derived from the two precursors exhibited CO2 uptake of 1.54 and 1.46 mmol/g at 298 K and 0.15 bar. Though the peanut shell derived porous carbon had much lower surface area and micropore volume than the sunflower seed shell derived porous carbon, it showed higher CO2 uptake owing to its higher volume of micropores in the range of 0.33–0.44 nm. Moreover, the volume of micropores with a width of 0.33–0.44 nm had a linear relationship with the CO2 uptake, demonstrating that these micropores were responsible for CO2 adsorption. Many more studies reported that porous carbons with a pore size of 0.7–0.9 nm possess the maximal CO2 adsorption capacity rather than the larger pores (d > 1 nm) [43,112,113,114]. Recently, Liu et al. [115] further identified that the advantageous ultramicropores (0.6–0.8 nm) in N-doped biochar played a decisive role in CO2 uptake. The pore size in carbon can be roughly controlled by adjusting the carbonization temperature and activation process, while the design and synthesis of efficient porous carbon with a suitable micropore size requires more sophisticated means, which include choosing a proper precursor, performing an appropriate pre-treatment method and activation process.
Tang et al. [116] prepared porous carbon by selecting date sheets as carbon sources, combined with controllable carbonization and subsequent KOH activation. Figure 7a shows the synthesis procedure. The date sheets have the natural internal open pores consisting of thin laminates, which can be tailored towards promoting the activation step. The fresh date was first pre-treated with washing, impurity removing and cutting into small sheets, and the reprocessed date sheets were freeze-dried overnight for 48 h and designated as FDDS (freeze-dried date sheets). The FDDS were carbonized at 500 and 800 °C obtaining CDS-500/800, and the CDS were activated by pyrolysis of the mixture of CDS/KOH with a certain ratio to achieve ACDS. Figure 7b,c display the N2 isotherms and corresponding PSD plots. These isotherms and PSDs demonstrated that the pore structure varied significantly with the mass ratio of KOH to CDS. All the samples have type-I isotherms with a high N2 uptake at a low relative pressure (P/P0 < 0.1) and a wide adsorption knee and plateau, indicative of the microporous structure. As the KOH/CDS increased from 2 to 6, the adsorption knee became wider, suggesting the production of more mesopores. This study disclosed that the temperature had less impact on the pore structure, as the temperature increased from 500 to 800 °C, the N2 isotherms, PSDs and the textural parameters did not change much, maybe due to the carbon source and freeze-dried pre-treatment. Figure 7d exhibits the CO2 adsorption isotherms over all the date derived carbon samples. It can be observed that the CO2 adsorption capacities of all activated samples are obviously higher than those of the mere carbonized samples without activation. The ACDS-500-2 has a CO2 adsorption capacity of 5.98 mmol/g, which is close to ACDS-800-2 and ACDS-800-4 with values of 6.0 and 6.4 mmol/g at 0 °C. Figure 7e shows the typical adsorption isotherms toward CO2 and N2 at 25 °C on ACDS-800-4, demonstrating the good selectivity (41.53) of ACDS-800-4. The relationship between the pore size and CO2 uptake in Figure 7f revealed that a good linear relationship existed as the pore size was in the range of 0.7–0.9 nm, and the pores with a size of 0.5–0.7 nm play a major role in CO2 adsorption. The adsorption of CO2 has the optimized energy [117], when the pore size is two or three times of a CO2 molecule (ca. 0.33 nm).
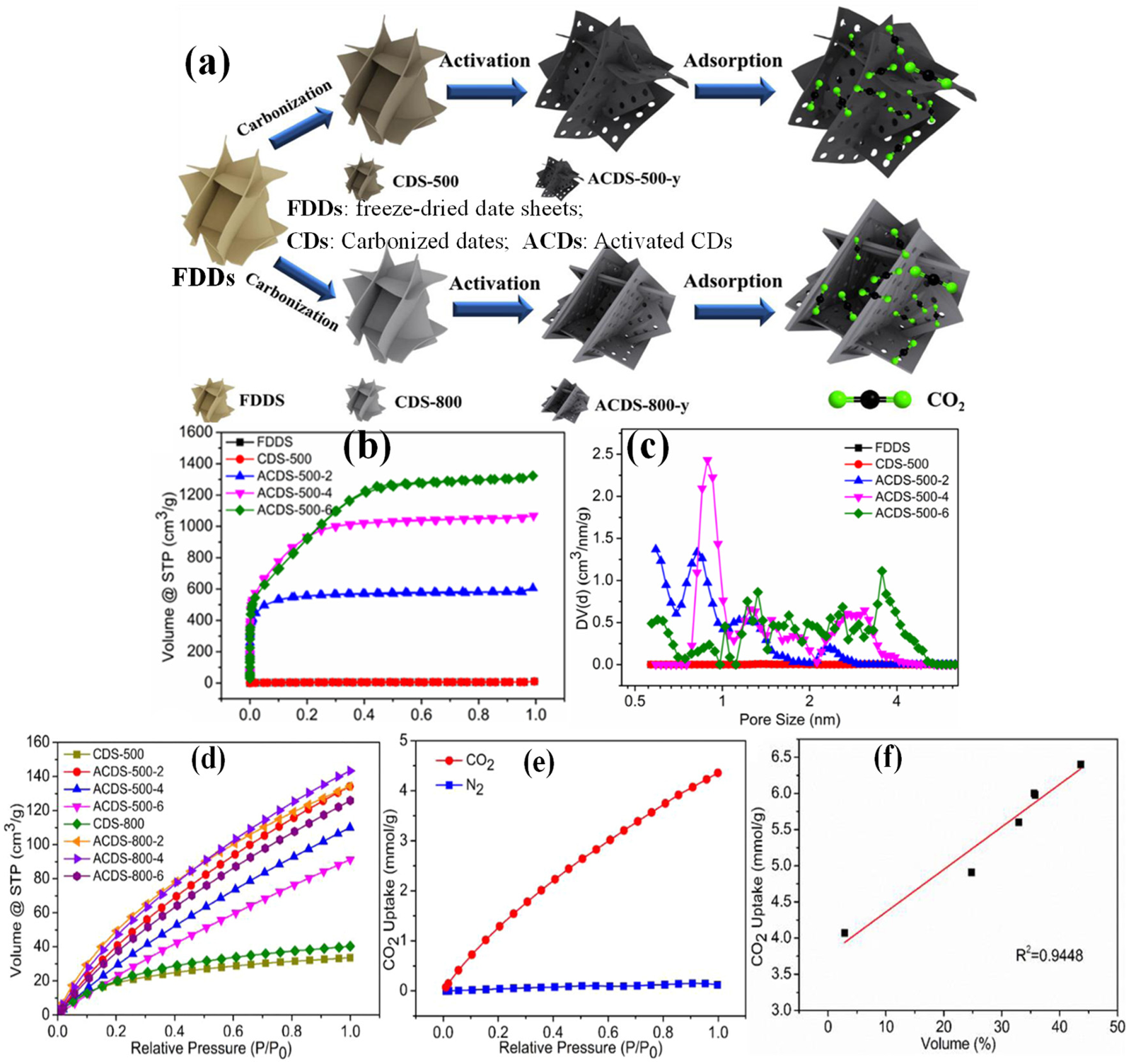
Figure 7. (a) Illustration of the syntheses of CDS-x, ACDS-500-y and ACDS-800-y from FDDS; (b) N2 adsorption/desorption isotherms and (c) pore size distributions (PSDs) plots of FDDS, CDS-500; (d) CO2 adsorption isotherms of all samples at 0 °C and 1 bar, (e) CO2 and N2 adsorption isotherms at 25 °C and (f) Correlations between the amount CO2 uptake and proportion of cumulative pore volume of pores with pore size of 0.7–0.9 nm of ACDS-800-4. [Reproduced with permission from ref. [116]. Copyright 2019, Elsevier].
Up to now, most studies focused on enhancing the CO2 gravimetric adsorption performance onto porous carbons with low packing density, which can cause the insufficient utilization of pores rich in porous carbons. On the other hand, CO2 adsorption capability per volume of porous carbons can provide a relatively realistic indication of their performance, compared with the gravimetric performance. Additionally, the volumetric CO2 adsorption capability of porous carbon is an essential indicator for assessing the efficiency and scale of adsorption systems [118,119,120], whereas it is often overlooked in the field of CO2 capture by adsorption [102,121]. For the sake of obtaining porous carbons with high volumetric CO2 adsorption capacity, porosity regulation of carbon is a prerequisite because the pore configuration and size can influence remarkably the packing density of porous carbons [122]. In order to develop desirable porous carbon with high volumetric CO2 adsorption capacity, Qie et al. [123] employed a simple chemical activation using K2CO3 as the activating agent to prepare coal-derived porous carbons. Figure 8a displays the fabrication process of coal-derived porous carbons and the formation mechanism. By simply varying the pyrolysis temperature from 600 to 900 °C, the porosity in the coal-derived carbons can be controlled. As illustrated, the ultra-micropores obtained at 600–700 °C in the resulting porous carbons gradually grew into micropores and meso-micropores at 800 and 900 °C, respectively. Figure 8b,c shows the N2 adsorption-desorption isotherms and the correlation between the CO2 uptake and the PSDs. The obtained ACs were used as a CO2 adsorbent, revealing the importance of ultramicropores for CO2 uptake. The ultra-microporous carbon (AC-600) with a high packing density exhibited the highest volumetric capacity of 1.8 mmmol/cm3 at 0 °C. Their experimental results and molecular dynamic simulation disclosed that CO2 adsorption capacities increased linearly with the micropore volume, whereas the volumetric capacities were directly proportional to the ultra-micropore volume.
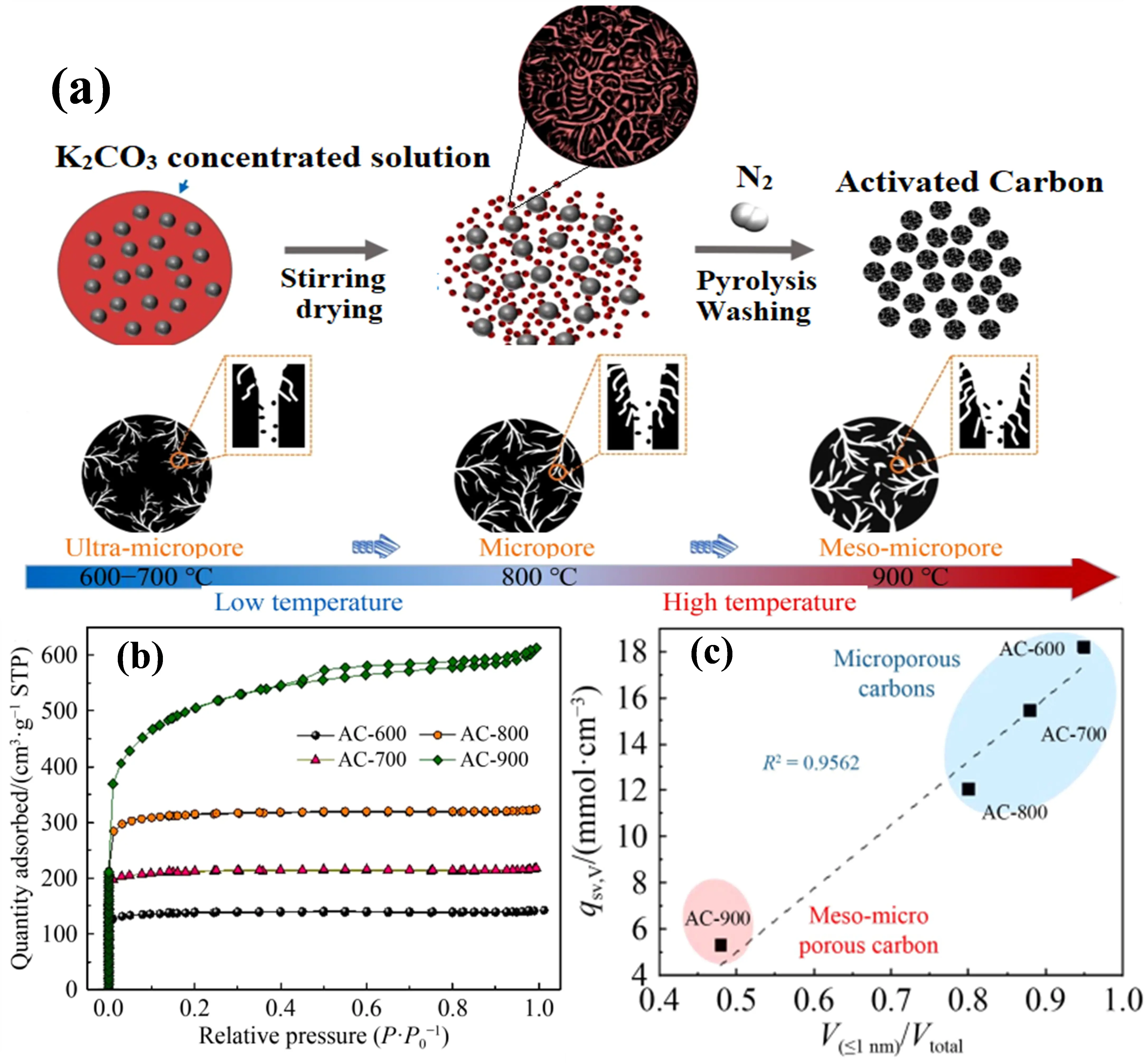
Figure 8. (a) Schematic illustration of the fabrication of coal based activated carbon (AC) by K2CO3 activation and the corresponding pore formation mechanism in the ACs; (b) N2 adsorption/desorption isotherms of the ACs prepared at different activation temperatures; (c) Correlations between the amount CO2 uptake per volume and V(d ≤ 1 nm)/Vtotal) of the ACs at 0 °C. [Reproduced with permission from ref. [123], Copyright 2022, Springer].
Chemical activation has advantages in preparing porous carbons, for example, the obtained porous carbons usually have a high specific surface area and abundant micropores, and the operating temperature is low (<900 °C) [124]. On the other hand, it has obvious shortcomings, which are the use of strong alkali or acid in the activation process, which will cause low carbon yield, corrosion to the instruments and environmental pollution. Apart from chemical activation, physical activation is often used to tune porosity in carbon materials. Physical activation has been well commercialized for the production of coal or biomass derived porous carbon. Specifically, in the physical activation process, oxidizing gases (i.e., H2O, CO2, O2, etc.) are often used as the activation agents to generate pores, in which small volatile molecules first release from the carbon matrix forming the initial pores at low-/medium temperature, then the gas activation agents gradually etch the carbon framework from the surface to inside to create desirable porosity. This process usually involves the reactions: C + H2O → H2 + CO (−131 kJ) or C + CO2 → 2CO (−170.5 kJ) at varied temperatures (ca. 700–1000 °C) [124,125]. Relatively, physical activation is environmentally friendly, while it wastes a lot of carbon. The main strategy of regulating the pore structure of carbon by physical activation is to adjust the reaction atmosphere [126], for example, choosing a proper activating agent and temperature, due to the diversity in molecular size, diffusivity and reactivity of the activation agents. Recently, microwave pyrolysis has been shown to be an efficient alternative for fabricating porous carbons with uniform pore structures and high specific surface areas, owing to its inward-to-outward heating mode, which reduces pore blockage and promotes pore development [127]. However, the porous carbons obtained by physical activation generally have low specific surface area (less than 1000 m2/g) and low pore volume (<0.5 cm3/g), because of the low reactivity between gaseous activation agents and carbon framework, so the adsorption performances are usually unsatisfactory.
In view of the weaknesses of a sole physical or chemical activation method, it is necessary to develop low-cost, facile and effective strategies for tuning porosity in carbon. Catalytic activation is a promising method, referring to the addition of metal compounds to a carbon source to increase the surface-active points. To tune the porosity of coal-derived carbons and reduce the dosage of activation agents, Sun et al. [128] proposed a green and efficient strategy for preparing porous carbons, namely, a trace K2CO3 induced catalytic CO2 activation system. In their strategy, a small amount of K2CO3 was added (less than 2% weight ratio of coal), which can prominently reduce the reaction barrier between the CO2 molecule and the coal framework, facilitating the pore formation of the coal-derived porous carbons. The resulting coal-based porous carbon using trace K2CO3 has short range ordered microcrystalline structure and developed pore structure with a high specific surface area of 1773 m2/g and pore volume of 1.11 cm3/g, even superior to the porous carbon using a large dosage of K2CO3 (K2CO3/C=3), and was three times more than those samples from solely CO2 activation. They found that the potassium-base component (mainly C–O–K structure) embedded in the coal matrix can act as a catalyst for intense erosion reaction between coal and CO2 molecule, which was generated during a CO2 atmosphere at high temperature and can enhance the pore formation in the resulting porous carbons. When used as a CO2 adsorbent, the trace-K2CO3 catalyzed porous carbon exhibited high CO2 adsorption capacity with value (4.36 mmol/g at 0 °C) and good adsorption-regeneration cycling stability.
4.2. Surface Modification
In addition to textural properties, the surface chemical structure of porous carbon has been verified to affect CO2 adsorption capability significantly [126,129,130,131,132]. The chemical property depends on the type and number of surface functional groups, which affect the interaction between CO2 and the porous carbon. As discussed above, the adsorption capacity toward CO2 will decrease with increasing temperature, but can be enhanced prominently by chemical modification. For example, when basic groups are introduced into the surface of porous carbon, the modified porous carbon will show stronger interaction with CO2 than the pure porous carbon, due to the introduced basic sites having intermediate affinity to CO2. Of course, the affinity is inadequate to induce chemical adsorption for CO2 [133]. The enhancement of the interaction between the adsorbent and CO2 molecules can be realized by various kinds of surface modification techniques. The modification techniques for porous carbon include physical, chemical or microbiological methods, which are very important because they can change the physical structural properties or surface chemical functional groups [134], so as to enhance the CO2-adsorbent interaction. The direction of these modifications is pore functionalization by introducing polar groups, for instance, nitro, hydroxy, amine, sulphonate, imine, etc. [129]. These surface functional groups (SFGs) can be introduced either prior to adsorbent synthesis by judicious selection of modification of the precursors, where CO2-philicmoieties will form during the synthesis procedure, or alternatively via post-synthesis modification when functional groups are introduced and attached to the surface of carbon.
Heteroatomic doping is a frequently used strategy to modify the surface chemical properties of pristine carbons. For most pristine porous carbons, their CO2 adsorption capacities are less than 3 mmol/g at atmospheric pressure [113], which is attributed to the limited active sites mainly derived from defects on the surface of adsorbents. Heteroatom doping usually involves the introduction of nonmetal element into the carbon matrix, and it is one of the most efficient ways to generate defects, in that the doped heteroatoms have similar atomic radius, orbit, electronegativity and charge density with carbon atoms that can change the electron and surface charges of the final materials [132,133,134]. Various kinds of heteroatoms have been successfully doped into porous carbons, for example, N, B, O, S, P, etc., obtaining higher CO2 adsorption capacity and selectivity compared with the unmodified counterpart [135]. The enhancement is primarily attributed to increased electrostatic interactions with acid-type gases, such as CO2.
As an adjacent element to carbon, nitrogen bears several similar chemical properties and atomic size with carbon [136], and can generate relatively stable chemical bonds with carbon [137]. Due to such similarities, introducing nitrogen will theoretically the electron density of the carbon framework or increase the basicity, which will in turn anchor the electron deficient carbon of CO2 to the carbon pore surface via Lewis acid-base (N atom) interactions [138]. Therefore, nitrogen is the most investigated heteroatom doped in carbon materials [139], since nitrogen atoms can easily enter the carbon skeleton without causing a serious lattice mismatch. Nitrogen-doped carbons generally refer to the modified carbon matrix where few carbon atoms are replaced by nitrogen atoms, being enriched with diverse types of nitrogen bearing functionalities on their surface. The possible nitrogen species and nitrogen functionalities formed on carbon surface are illustrated in Figure 9. The typical nitrogen species on carbon surface include pyridinic, pyridonic, pyrrolic, graphitic or quaternary nitrogen, combined with amine, nitro, nitroso and amide type. The amine functionalities consist of all the primary, secondary and tertiary amine. Apart from quaternary nitrogen, the other types of N-functionalities formed at the edge site of the graphitic plane, creating more defects than the undoped carbon.
Generally speaking, doping nitrogen via post-synthesis (activation) method will decrease the specific surface area and pore volume, for the introduced N in the carbon skeleton can lead to blocking of the earlier developed pore structure, which is disadvantageous to the CO2 adsorption capacity. However, the introduction of N can result in a considerable decrease in the amount of surface acidic groups and an increase of surface basic groups due to the generated nitrogen functionalities. Specifically, nitrogen functionalities on the carbon surface provide a lone pair of electrons, so they can act as the attractive sites for the electron-deficient carbon atom of the CO2 molecule owing to the high electron-withdrawing properties of oxygen atoms [136]. Moreover, the basicity of nitrogen-doped carbon can strengthen the dipole-dipole interactions and hydrogen- bonding to the surface, leading to an increase in the selectivity over non-polar gases such as N2 and CH4 [137,138]. On the other hand, the polar nitrogen functional groups will improve the hydrophilicity of carbon, and the H2O molecules are usually trapped inside the narrow micropore of carbon on account of the enhancement in hydrogen bonding with H2O [131,139,140]. Thus, the nitrogen-doped porous carbons may be better suited to the adsorption removal of CO2 in the dry flue gas.
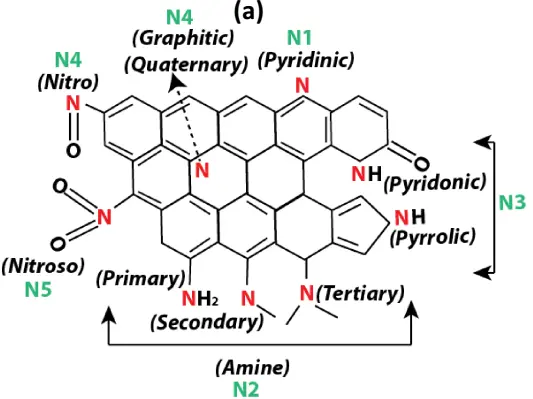
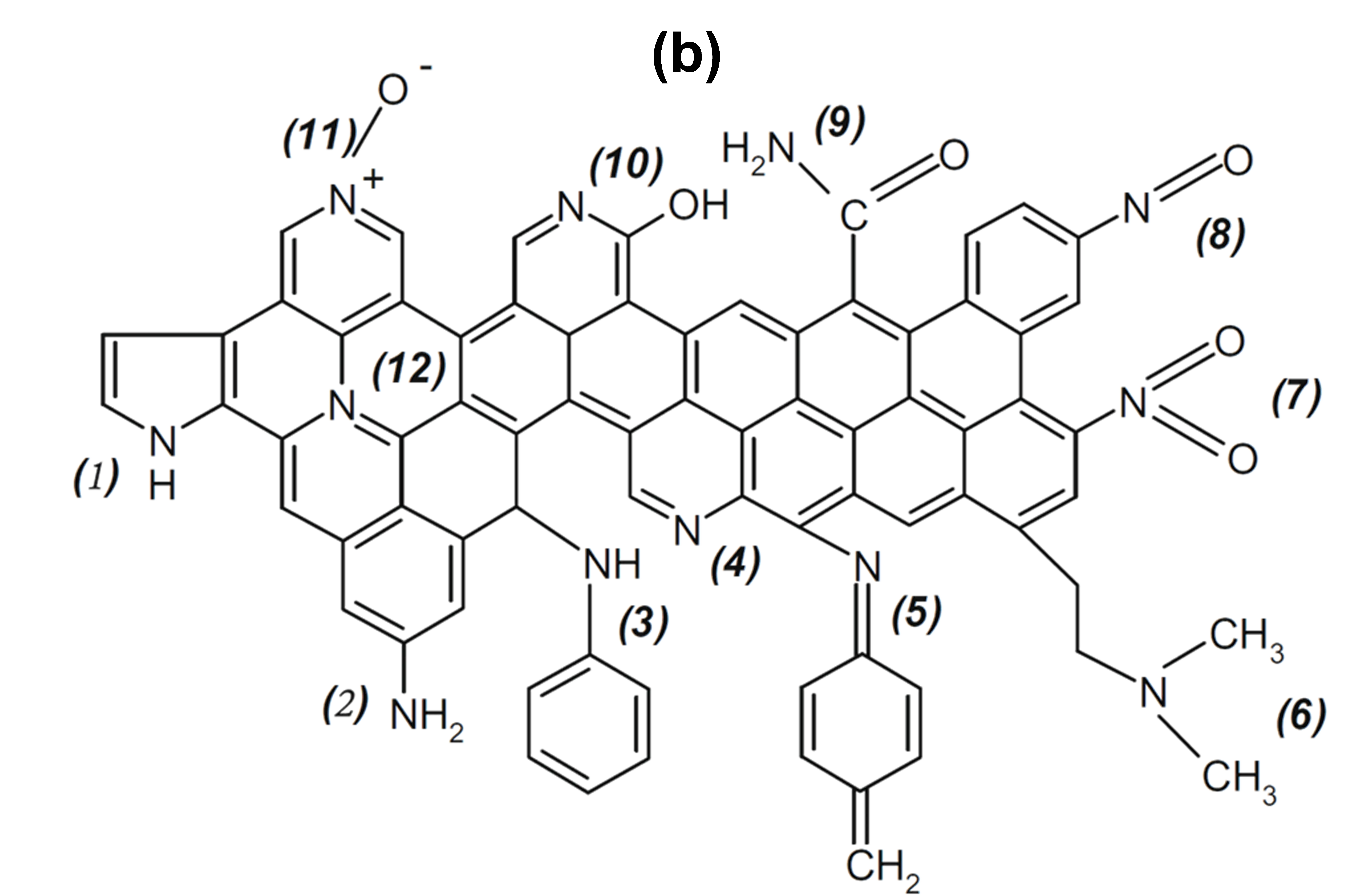
Figure 9. (a) Nitrogen species on doped carbon surface via NH3 activation (Reproduced from Ref. [131], with permission from Elsevier), (b) Various types of nitrogen functional groups on coal-derived nitrogen-doped carbon: (1) pyrrole, (2) primary amine, (3) secondary amine, (4) pyridine, (5) imine, (6) tertiary amine, (7) nitro, (8) nitroso, (9)amide, (10) pyridone, (11) pyridine-N-oxide, (12) quaternary nitrogen (Reproduced from Ref. [140], with permission from Elsevier).
Nazir et al. [141] fabricated N-doped porous carbons utilizing polyacrylonitrile (PAN) as carbon source and NaNH2 as both porogen and nitrogen supply during carbonization. The resulting optimized “PN-3” sample had satisfactory textural features with SSA of 2490 m2/g and pore volume of 2.06 cm3/g, together with nitrogen content of 6.4 at.%. The experimentally measured CO2 uptake for all the N-doped porous carbons ranged from 5.17 to 7.15 mmol/g at 273 K/1 bar, from 4.13 to 5.59 mmol/g at 283 K/1 bar. It also exhibited an excellent CO2/N2 selectivity (~102) based on the ideal adsorbed solution theory at 273 K, surpassing the performance of most undoped porous carbons. Additionally, the CO2 adsorption process was driven by physisorption, indicated by the relatively low isosteric adsorption heat of 39.10 kJ/mol and low activation energy of 5.05 kJ/mol. Wang et al. [142] synthesized nitrogen-doped porous carbons through carbonization of ZIF-8 and ZIF-61 assisted by KCl and NaCl, showing a highly microporous structure and high nitrogen content. Their results demonstrated that the cumulative pore volume in the pore size of 5–7 Å, pyrrolic-N content and COOH content played favorable influence on CO2 adsorption capacity. The evaluation by a multiple linear regression equation confirmed that the optimum pore structures (V5–7Å > 0.10 cm3/g) and pyrrolic-N were the dominant contributors to CO2 adsorption capacity. The highest CO2 adsorption capacity of 4.61 mmol/g at 298 K and 6.70 mmol/g at 273 K can be acquired with a high CO2/N2 selectivity of 39.5 under typical flue gas and cycling stability.
Besides PAN and ZIF, other precursors have been utilized to prepare nitrogen-doped carbon via in-situ method. Liu et al. [143] transformed oxytetracycline fermentation residue into an in-situ N-doped nanoporous carbon by low-temperature pyrolysis coupled with pyrolytic activation. They found that the mild activation conditions (600 °C, KOH/carbon = 2) resulting in micropore-rich porous carbon with high in-situ nitrogen content, and the doped nitrogen in the carbon skeleton with high oxygen enhanced electrostatic interactions facilitating the CO2 adsorption. The maximal adsorption capacity arrived at 4.38 and 6.40 mmol/g at 298 and 273 K and 1 bar, with high CO2/N2 selectivity (32/1) and favorable Qst (33 kJ/mol) as well as excellent reusability (decreased by 4% after 5 cycles). Wu et al. [144] produced nitrogen-doped porous carbons from bamboo shoot shells using urea as a nitrogen source, activated by potassium carbonate. Their results revealed that the as-produced N-doped porous carbon exhibited a fibrous structure and distinct worm-like microporous structure, and the largest specific surface area was 1985 m2/g with a nitrogen content of 1.98 at.%. The optimized sample demonstrated outstanding capacity of CO2 adsorption with values of 7.52 and 3.60 mmol/g at 273 and 298 K under 1 bar, and it also exhibited excellent cyclic stability and CO2/N2 selectivity. The thermodynamic calculations verified that the CO2 adsorption process on nitrogen-doped biochar was spontaneous and exothermic, indicative of its physical adsorption. Furthermore, Zhang et al. [145] utilized fir sawdust as a precursor, employing urea and thiourea as nitrogen and sulfur sources with a secondary activation strategy to yield micropore-rich carbon. The resulting material showed high selective CO2 capture with an uptake of 3.96 mmol/g at 298 K and 1 bar and a CO2/N2 selectivity of 47.
Like nitrogen, boron is an adjacent element to carbon with similar chemical properties and atomic size to carbon [141], so the spatial structure of B-doped carbon materials will not change significantly [142], while there are more active sites on the B-doped carbon surface with enhanced adsorption performance due to the uneven electron distribution on their surface [143]. By contrast, boron loses electrons while nitrogen gains electrons to form relatively stable chemical bonds with carbon (BC3), suggesting that the carbon atoms adjacent to boron show different electronegativity from those adjacent to nitrogen [146]. Li et al. [147] investigated the influence of boron-doped porous carbon on the CO2 adsorption performance. Their results demonstrated that the B atom played a beneficial role in the CO2 adsorption process, and the B-doped carbon with a B/C ratio of 0.05 exhibited the highest CO2 adsorption performance with 2.16 mmol/g at 303 K and 1 bar. Through in-depth analysis, it is found that boron presents some contradictions in the adsorption performance of B-doped carbons. On one side, boron doping can create useful active sites and the boron-doped carbon has some Lewis basicity, which is advantageous to the interaction with CO2 molecules having weak Lewis’s acidity. On the other hand, the oxygen affinity of boron means the boron-doped structure is easily oxidized, and the Lewis alkalinity of the oxidized structure is weakened that impair the CO2 adsorption performance. Thus, it requires further exploration of how to form stable boron-doped carbon for improving CO2 adsorption performance of B-doped carbon.
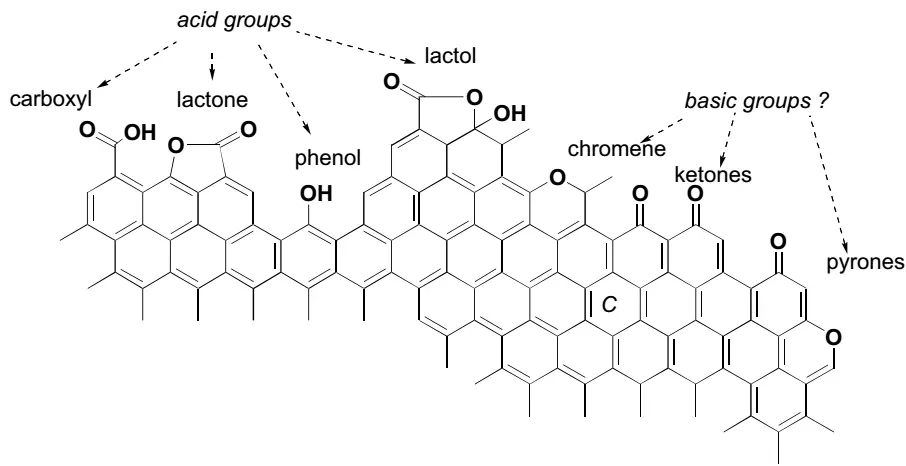
Oxygen is often doped into the carbon skeleton to improve the CO2 adsorption performance, because oxygen is the next most frequent element to carbon, and the oxygen-containing groups can contribute to determining the hydrophobicity/hydrophilicity and acidity/basicity of carbon adsorbents. Figure 10 shows the common oxygen-containing functional groups [146], which are traditionally divided into two categories according to their acidic or basic character. It is well-recognized that carboxyl groups, lactones, phenol and lactol groups account for the acidic character of carbon materials. While there are controversies on the basic groups, chromone, ketones and pyrones were proposed as the basic O-containing functionalities [147,148]. The pyrone-like groups at the edges of the carbon surface could be the most important basic functionalities. The pristine carbon is usually hydrophobic, so the degree of hydrophilicity is often decided by the amount of polar O-groups [130]. Unfortunately, the increase of hydrophilicity is not conducive to CO2 adsorption, since it enhances the adsorption of moisture in the flue gas, leading to the reduction of active adsorption sites toward CO2. Even so, the oxygen functionalities can improve the adsorption properties of carbon toward CO2. For example, an increase of 26% in CO2 uptake has been reported on the oxidized phenolic resin derived carbon compared with the unmodified carbon [149].
Generally, O-containing functionalities are produced from the oxidation of organic structures during various kinds of chemical/physical activation procedures [150]. Acidic groups are formed at relatively low temperatures under oxidization with HNO3, H2O2, H3PO4 or H2SO4 [151]. More strong surface basicity could be introduced by NaOH activation than H3PO4 at the same temperature of 850 °C. The basic groups can be more effectively introduced by activation with H3PO4 in the presence of steam than in an inert (N2) atmosphere [152], and extreme activation conditions were shown as detrimental to the formation of surface functional groups [153]. The surface O-containing groups of porous carbon have electron-rich properties, which can strengthen the interactions between the CO2 molecule and carbon surface owing to the introduction of various polar groups [154]. Bai et al. [155] modified activated carbon fibers (ACFs) by liquid oxidation with hydrofluoric acid to introduce O-containing functional groups. The liquid-oxidized ACF had 112% higher CO2 adsorption capacity than those observed onto the pristine ACFs. The enhancement was attributed to the synergistic effects of developed pores and the O-functionalities that guide CO2 into the micropores via the attractive forces exerted on the electrons in CO2 molecules. Then they concluded that the introduction of carboxylic and hydroxyl surface groups was effective in improving CO2 adsorption performance.
As for the enhancement of CO2 adsorption by oxygen-doped carbon, there are some different reports. Tiwari et al. [156] thought the high content of O-containing basic groups contributed to the highest CO2 adsorption capacity of the epoxy resin-derived carbons. Babu et al. [157] found aht the CO2 adsorption capacity increased with the oxygen concentration, and postulated that both –OH and –COOH groups facilitated the considerable improvements in CO2 adsorption capacity at ambient pressure. As mentioned above, nitrogen-containing groups can present Lewis basic character of carbon materials. Beyond that, the oxygen-containing groups also have a similar function. O-groups like ethers, alcohols and carbonyls comprise electron-donating oxygen atoms, which participate in the electrostatic interactions with the CO2 molecule [149,158], then improving the adsorption of polarizable species such as CO2 by Lewis acid-base interactions [159]. Among the basic oxygen functionalities, pyrone is often investigated, and pyrone-like structures are the combinations of non-neighboring carbonyl and ether-O at the edges of a graphene layer [146]. The basicity of pyrone originated from the stabilization of its protonated form via electronic π-conjugation throughout sp2 skeleton of the carbon basal plane [130,160]. –OH group is often reported as basic and affects the adsorption of CO2 due to its high electron density [161,162]. The interactions between –OH groups and the CO2 molecule mainly depended on the hydrogen-bonding and high electrostatic potential [163,164]. A linearity between CO2 adsorption capacity and the content of oxygen groups was observed at 298 K below 0.5 bar, while it was not found when the adsorption pressure was increased from 0.5 to 1 bar.
Sulfur is also frequently doped in carbon materials to improve their application performances. Compared with N, O and B atoms, sulfur is much larger in size so that it can protrude out of the graphene plane, resulting in the increased graphite layer spacing, the distortion and deformation and a large amount of strains and defects within the carbon skeleton [130,165]. These defects and strains can alter the original charge distribution, inducing charge localization, so that more active sites will be produced in the S-doped carbons that are conducive to CO2 adsorption [166]. Additionally, S-doping can increase the reactivity owing to the lone-pair electron donation of the sulfur atom [167]. Xia et al. [168] reported that the S-doped carbons exhibited higher CO2 uptake capacity than the S-free carbon, which was ascribed to the strong pole-pole interactions between the large quadrupole moment of CO2 molecules and polar S-groups associated with sites. Seem et al. [169] found a linear relationship between CO2 adsorption capacity and the quantity of oxidized-S in the S-doped porous carbons, and the S-doped microporous carbons were more effective than similarly prepared N-doped samples in CO2 adsorption. Density Function Theory (DFT) calculations revealed that the interaction of CO2 with sulfoxides and sulfones was stronger than those with N-moieties, according to the calculated binding energy.
Phosphorus is another heteroatom extensively doped in carbon materials. Phosphorus belongs to the nitrogen group and is also electronegative, having similar chemical property with nitrogen, doping phosphorus into carbon will alter the electron density of carbon structures, producing some active sites [170,171]. Different from nitrogen, phosphorus has a larger atomic size, thus the formation of the C–P bond will increase the spacing of the graphite layer, leading to further changes in density [172]. Up to now, there are few reports on phosphorus dope carbon materials applied in CO2 adsorption, so the corresponding enhanced adsorption mechanism is rarely reported and not clear. Zhou et al. [171] investigated the CO2 capture by several carbon phosphides with the assistance of an electric field, finding that the carbon phosphide acting as a CO2 adsorbent had good CO2 capture performance. However, a recent study on N and P co-doped porous biochar has successfully demonstrated enhanced CO2 capture performance under conventional conditions and provided a detailed synergistic mechanism analysis focusing on the tuning of nitrogen species and active sites [173]. Accordingly, it is necessary to conduct further investigations to understand how the atomic charge of phosphorus and the spatial structure of phosphorus doped carbon affect the CO2 adsorption.
4.3. Metal Impregnation
Another modification of porous carbon is metal modification, and numerous reports have clearly indicated that the impregnation of metal ions onto carbon can combine their respective multiple advantages, resulting in great-enhanced CO2 adsorption performance [174,175,176]. In fact, metal oxides are often employed as adsorbents independently for CO2 adsorption in industrial applications, due to their acid-base and redox properties. Unfortunately, there are some limitations for the usage of metal oxide alone in CO2 adsorption, for example, low surface area and poor stability. Impregnating metal oxide can increase the oxygen groups on the surface of porous carbons, serving as a metal oxide anchoring site, which facilitates the CO2 adsorption by tailoring the basic characteristics of porous carbons. Metal oxide impregnated porous carbon (MPC) has excellent mechanical features due to the size quantization effect, especially towards CO2 [177], and a great number of active sites for CO2 adsorption will be generated. Therefore, MPCs have been employed to improve CO2 adsorption ability; various kinds of metals, including sodium (Na), Calcium (Ca), magnesium (Mg), nickel (Ni), etc. have been introduced into porous carbons for improving CO2 adsorption capability of porous carbons [153,178].
MPC can effectively adsorb CO2 via physical and chemical sorption over a wide temperature from 473 K to 673 K, and low energy is required for CO2 regeneration [179]. Othman et al. [180] incorporated four different types of metal oxides into activated carbon nanofibers (ACNF), such as magnesium oxide (MgO), manganese dioxide (MnO2), zinc oxide (ZnO) and calcium oxide (CaO) by electrospinning and pyrolysis process. They observed the ACNF with MgO incorporation exhibited the largest surface area of 413 m2/g and the highest CO2 adsorption of 60 cm3/g at 298 K and 1 bar. Similarly, Lahijani et al. found that a Mg based MPC showed a higher CO2 adsorption potential (82.0 mg/g) than raw activated carbon (72.6 mg/g) [153]. In addition, cyclic CO2 capture experiments demonstrated that porous carbon containing basic metals had a higher capacity for CO2 adsorption because it can react with acidic CO2 molecules. MPC impregnated with Mg via Mg(NO3)2 salt yielded 3.1 mmol/g CO2 adsorption, while MPC with Mg-sugarcane via MgCl2 salt achieved CO2 adsorption capacity of 227–235 mg/g [176]. The plain TiO2 showed a comparably limited CO2 adsorption capacity verified by the isothermal experiments [181], and the highest CO2 adsorption capacity was only 3.1 mmol/g at 313 K, even up to 25 MPa. Likewise, Tungsten (VI) oxide (WO3), as an earth-abundant metal, has a low CO2 adsorption capacity of 0.3 mmol/g at 313 K that is lower than that of TiO2 [182]. Fortunately, impregnating TiO2 or WO3 into carbon can improve the CO2 adsorption affinity, as the doping procedures and composites of metal oxide-carbon increase the Lewis basicity, allowing their better adsorption of Lewis acidic CO2 [183]. Especially, the addition of an oxygen-rich surface functionality of porous carbon to the WO3 lattice can improve the CO2 adsorption, implying a better affinity toward CO2.
Table 2 summarizes the porous carbons modification with impregnation of various kinds of metal oxides, for example, MgO, NiO, CuO, etc. All these results have indicated that the impregnation of metal oxide onto porous carbon surely enhanced the CO2 adsorption capacity compared with the raw carbons. On the other hand, there are also some reports that the CO2 adsorption capacity was reduced as metal oxides were impregnated into porous carbons.
Table 2. Summary of CO2 adsorption performance of porous carbon impregnated with different metal oxides.
|
Adsorbent Materials |
SBET (m2/g) |
Adsorption Conditions |
CO2 Uptake (mmol/g) |
Change of Perf. (%) |
Refs. |
||
|---|---|---|---|---|---|---|---|
|
Carbon Precursor |
Metal Oxides |
Temp. (K) |
Pres. (bar) |
||||
|
Coconut shell |
MgO |
976 |
298 |
1 |
2.63 |
50.3 |
[184] |
|
Commercial Activated carbon |
NiO |
925 |
298 |
1 |
2.73 |
10.1 |
[185] |
|
MgO |
820 |
298 |
1 |
2.68 |
8.1 |
||
|
Activated carbon |
CuO |
1974 |
303 |
1 |
6.78 |
124.5 |
[186] |
|
Mesoporous carbon |
MgO |
298 |
353 |
1 |
5.45 |
- |
[187] |
|
Charcoal |
CaO |
63.6 |
298 |
1 |
15.1 |
- |
[188] |
|
Carbon nanofiber |
NiO |
331 |
298 |
1 |
2.40 |
- |
[189] |
|
Hickory chips |
FeOOH |
- |
298 |
1 |
3.64 |
much |
[190] |
|
Activated carbon |
Cu/Zn |
599 |
303 |
100 kPa |
2.25 |
48.0 |
[191] |
|
Walnut shell |
MgO |
292 |
298 |
1 |
1.86 |
12.9 |
[153] |
|
Carbon nanofiber |
MgO |
413 |
298 |
1 |
1.38 |
- |
[180] |
|
Activated carbon |
γ-Fe2O3 |
833 |
298 |
1 |
2.47 |
27.1 |
[192] |
|
Activated carbon |
CuO |
1277 |
303 |
100 kPa |
2.11 |
28.7 |
[193] |
Li et al. [194] modified porous carbon by multiple metal oxides, such as CeO2, CuO, NiO and Mn3O4 with varied weight loadings of 10–30%. They investigated them as adsorbents for CO2 capture, observing that the overall CO2 adsorption capacity decreased upon metal oxide modification. They attributed this to the reduction in the total surface area of the porous carbon/metal oxide composite. As a matter of fact, the surface area of metal oxide is far below that of porous carbons, so the total surface area of the composite will usually be decreased due to the covering of the surface by metal oxides, especially under an excess amount of metal oxides impregnated. Luckily, the CO2 adsorption capacity of MPC at a relatively low-pressure range (below 0.4 bar) increased with high metal oxide loading (30%), particularly for NiO modified MPC, owing to the stronger interaction strength between CO2 and metal oxides than with the carbon surface. In addition, the CO2/N2 selectivity values of MPC are generally higher than those of the pristine porous carbons, and the 30% NiO-modified MPC exhibited 72% increase in the selectivity, which is ascribed to an increased interaction between CO2 and the introduced metal oxides supporting by the increased isosteric heats of CO2 adsorption for MPC. The decrease in CO2 adsorption capacity onto MPC was also observed on ACNF@metal oxide composites. Othman et al. [180] impregnated MgO, MnO2, ZnO and CaO into ACNF via electrospinning method. They found that only MgO impregnated MPC showed slightly higher CO2 adsorption capacity than that of pristine ACNF, due to the decrease of Smicro percentages in these MPCs.
As a composite, the adsorption performance of MPC is inevitably determined by the dosage of metal impregnated on the MPC, especially for the ratio and type of metal precursor, as well as the amount of MPC in the CO2 adsorption process. Generally, metal impregnation modifies the surface chemistry of parent carbons, and the active adsorption sites will increase when metal is incorporated into an adsorbent. However, as the concentration of metal is beyond the ideal value, the active sites on carbon become saturated, the redundant metal cannot be bound and will block the pores in the parent carbon [195], resulting in the decrease of surface area, which will weaken the CO2 adsorption capacity of MPC. Thus, the loading amount of metal or the ratio of metal impregnated to the parent carbon is a key factor to produce an ideal MPC with appropriate pore size and abundant pores for the adsorption of the CO2 molecule. It has been verified that the porosity of MPC was inversely proportional to the loading amount of metal ion supplied [191]. Moreover, different kinds of metal show diverse affinity towards the CO2 molecule [196], so the MPCs with different metals have distinct adsorption performance. For example, the adsorption capacity of CO2 on metal-impregnated AC followed the order of Fe(III) > Mg(II) > Cu(II) > Ag(I)/AC. The incorporation of Fe and Mg enhanced the bonding with AC because Fe3+ and Mg2+ are hard acids, while the addition of Ag onto AC weakened the CO2 adsorption for Ag+ is a soft acid.
Apart from the ratio and type of metals, the metal precursor used in the adsorbent preparation also has important effects on the adsorption performance of the resultant MPC. Guo et al. [197] found that the hydromagnesite derived Mg-impregnated MPC exhibited lower CO2 uptake than that of the organometallic magnesium precursor, because there existed other compounds in hydromagnesite derived MgO, such as SiO2, CaO, Fe2O3 and Al2O3, which might block the active sites for CO2 adsorption [198]. Furthermore, the surface area, crystalline size and the impurities in the adsorbent can influence the adsorption capacity. The hydromagenesite derived MgO-impregnated MPC possessed flake and sheet-like geometry with a uniform microparticle dispersion, while the organometallic magnesium precursor derived MPC had a larger specific surface area and pore volume, smaller crystallite size and nanoparticle dispersion resulting in a better CO2 adsorption performance. In a word, the amount/ratio and type of metal precursor must be chosen prudently to achieve an ideal CO2 adsorption capacity for MPC, because they determine the morphology, crystallize size, surface area, pore volume and size, which play crucial roles in attaining the optimal CO2 adsorption performance.
The adsorption process towards CO2 onto MPC can be summarized into five sequential phases. Specifically, it consists of these following diffusion and adsorption steps: (1) bulk diffusion during which CO2 diffuse from concentrated gas phase to the surface of MPC; (2) Film diffusion where CO2 molecule diffuse from the gas film; (3) Interparticle diffusion where the adsorbed CO2 molecules diffuse into the pores of MPC; (4) Intraparticle diffusion where the adsorbed CO2 molecules diffuse into the crystalline grains of MPC; (5) Surface adsorption when CO2 molecule react or interact with the surface/functional groups of MPC [199]. Generally, the fifth step is very rapid, and the correlated resistance is found to be minimal [199]. The actual CO2 adsorption onto MPC occurs on the basis of these steps, sometimes parts of the adsorption sequence occur, and sometimes, a combination of these sequences occurs. The adsorption of CO2 onto MPC also mainly depends on physisorption via van der Waals force with a low heat of adsorption ranging from −25 to −40 kJ/mol, which is comparable to the sublimation heat of MPC [34]. For Ca-modified biochar, the isosteric heat of adsorption for CO2 ranged from 29 to 33 kJ mol−1, indicating a predominant physisorption mechanism with moderate strength, consistent with its excellent regeneration stability (93% capacity retention after five cycles) [200]. Physisorption is known as an exothermic process with low enthalpy value owing to the weak van der Waals attraction forces. The amount of molecules adsorbed is connected with the topological dimension of carbon pores and the pore volume of MPC [201], while it is inversely proportional to the number of pores filled with the metal oxide. Besides physisorption, chemisorption also plays an important role in the CO2 adsorption onto MPC. As illustrated in Figure 11, physical adsorption, chemical adsorption and hydrogen bonding synergistically contributed to the adsorption of CO2 onto Mg-impregnated mesoporous carbons. Liu et al. [187] disclosed the adsorption mechanism of CO2 on Mg-MPC by FTIR and XPS analyses. Based on the results, they concluded that the high CO2 adsorption capacity (5.45 mmol/g) cannot be solely ascribed to the surface area; the special functional groups on MPC-MgO also played an important role. According to the XPS spectra before and after adsorption of CO2 indicated, the O2− at the edges and corners of the MgO crystal were the main active sites for CO2 capture.
As displayed in the Figure 12, Azmi et al. [184] proposed the adsorption mechanism of Mg-impregnated hydrochar, offering insights into the interactions governing the phenomenon of enhanced CO2 adsorption. The process also involves CO2 binding to the carbon and carbon@MgO surfaces, and the adsorption is a combination of physical and chemical interactions. Nitrogen functional groups act as Lewis bases, forming coordinate covalent bonds with CO2 molecules. Simultaneously, the MgO contributes to the Lewis acid sites, facilitating chemisorption and the formation of carbonate species on its surface. In addition, hydrogen bonding plays a significant role between the oxygen atoms of CO2 and nitrogen functional groups. The porous structure of porous carbon boosts the physical adsorption via van der Waals forces. The positively charged sites on MgO and partial charges on CO2 result in electrostatic attractions, which further contribute to the overall adsorption process, enhancing the adsorption capacity. In summary, the synergistic effects of MgO, nitrogen functionalities and the porous structure of porous carbon create a multifaceted adsorption system. These multiple interactions highlight the complexity of CO2 adsorption onto this composite adsorbent, along with the hydrogen bonding and electrostatic/diffuse adsorption that play essential roles in the comprehensive adsorption mechanism.
4.4. Other Strategies
Recently, an activated carbon based “charged-adsorbent” was developed to capture CO2 effectively. As displayed in Figure 13, the preparation of charged-adsorbents is on the basis of charging of an electrochemical energy storage device [202], in which the electrolyte ions accumulate in the pores of a conductive porous carbon electrode during charging. After the completion of the charging process, the electrode is removed from the cell and washed/dried to yield a charged-adsorbent material. It is hypothesized that the accumulated ions in the porous electrode can serve as active sites for the adsorption of CO2. The resulting charged porous carbon can rapidly capture CO2 from ambient air by means of (bi)carbonate formation, showing enhanced CO2 uptake at low pressure, and can be regenerated at low temperature of 90–100 °C by direct Joule heating. Given the highly tailorable pore environments and low cost, in which the electrolyte and electrode can easily be adjusted, this charged-adsorbent is expected to have numerous potential applications in separations, catalysis and beyond.
Traditionally, capturing CO2 by adsorption onto porous carbons mainly depends on the three-dimensional (3D) pores, while two-dimensional (2D) pores in graphene are ideally suited for ambitious high-performance carbon capture. Hsu et al. [203] developed a strategy for improving the carbon capture performance by increasing the binding affinity of CO2 with the 2D pore. They incorporated pyridinic nitrogen at the pore edges by a simple exposure of ammonia to oxidized single-layer graphene at room temperature. The resultant highly selective 2D pores in graphene led to improved separation performance compared with other membranes for carbon capture. The pyridinic-N-substituted graphene pores can readily adsorb CO2 even from a very dilute mixture. An attractive combination of CO2/N2 selectivity (average of 53) and CO2 permeance (ca. 10420 GPU) was achieved, which was attributed to the high affinity combined with the 2D nature of the pores.
As we all know, the CO2 adsorption performance of porous carbon is primarily determined by its texture and surface chemistry, along with the doped heteroatoms and impregnated metal oxides. However, the complicated interplay between the textural and chemical properties of carbon prevents the comprehensive understanding of their respective contributions to CO2 adsorption capacity and selectivity. Eichler et al. [204] employed a dual cation activation method to control the fine pore structure of carbon independent from its surface N-content. By this method, the surface area can deviate significantly from 2200 to 4500 m2/g (note: values exceeding ~2630 m2g−1, the theoretical maximum for both sides of a graphene sheet, may arise from measurement artefacts or model-dependent estimations in highly microporous carbons) at a constant total nitrogen content of 2.3 ± 0.3 at%, or the surface area changed less from 400 to 675 m2/g at 23.1 ± 0.1at% N derived from nitrogen-rich precursor. From these carbons with variable porosity and constant heteroatom content, not only can the nature of the different adsorption sites be uncovered, but also the fundamental linear free energy exchange relationship can be established accordingly. Their results indicated that selectivity is almost entirely a function of relative porosity for physisorptive carbons, while the surface chemistry plays a negligible role in physisorption of either CO2 or N2 at the relevant flue gas conditions. The principal adsorption site is correlated with the narrowest pores that can accommodate a CO2 molecule (pore width < 0.37 nm).
A comparative summary of the CO2 adsorption performance for representative porous carbons developed via different strategies is provided in Table 3, highlighting the effectiveness of each approach under specified conditions.
Table 3. Comparison of CO2 adsorption performance of various modified porous carbon-based adsorbents.
|
Precursor |
Modification Strategy |
SBET (m2·g−1) |
Pore Volume (cm3·g−1) |
Adsorption Conditions |
CO2 Uptake (mmol·g−1) |
Selectivity (CO2/N2) |
Key Feature |
Refs. |
|---|---|---|---|---|---|---|---|---|
|
Rice husk |
KOH activation |
1496 |
0.786 |
273 K, 1 bar |
5.83 |
9.5 |
High micropore volume |
[41] |
|
Coal |
K2CO3 activation |
1703 |
0.83 |
273 K, 1 bar |
4.80 |
N/R |
High volumetric capacity |
[122] |
|
ZIF-8 |
N-doped carbon (KCl/NaCl) |
1000 |
0.40 |
273 K, 1 bar |
6.70 |
39.5 |
High pyrrolic-N content |
[141] |
|
Bamboo shoot shell |
N-doping (urea, K2CO3) |
1985 |
0.84 |
273 K, 1 bar |
7.52 |
N/R |
Fibrous, worm-like pores |
[143] |
|
Oxytetracycline residue |
In-situ N-doping, KOH |
1300 |
0.55 |
273 K, 1 bar |
6.40 |
32.0 |
Waste-derived, in-situ N |
[142] |
|
Fir sawdust |
N, S co-doping |
1400 |
0.60 |
298 K, 1 bar |
3.96 |
47.0 |
Secondary activation |
[144] |
|
Coconut shell |
Molten salt (NaOH-Na2CO3) |
3313 |
1.67 |
273 K, 1 bar |
6.02 |
N/R |
Ultra-high SSA, N/O co-doping |
[109] |
|
Hickory chips |
FeOOH impregnation |
N/R |
N/R |
298 K, 1 bar |
3.64 |
N/R |
Metal-biochar composite |
[189] |
|
Walnut shell |
MgO impregnation |
292 |
0.15 |
298 K, 1 bar |
1.86 |
N/R |
Metal oxide enhances chemisorption |
[152] |
N/R: Not Reported.
Moreover, beyond the initial adsorption capacity, the long-term cyclic stability and efficient regenerability of these modified porous carbons are paramount for practical deployment. For porosity-tuned carbons, physical adsorption is highly reversible, but pore blockage by impurities in real flue gas can gradually degrade performance. Surface modification via heteroatom doping, while enhancing affinity, must ensure the chemical stability of the introduced functional groups (e.g., N-species) over repeated adsorption-regeneration cycles, often involving thermal or pressure swings. Metal impregnation presents more complex challenges, as metal species may sinter, leach, or form stable carbonates, leading to irreversible capacity loss and increased energy demand for regeneration. Therefore, future research on these enhancement strategies must systematically incorporate rigorous cycling tests (e.g., >100 cycles) under realistic conditions, reporting not only capacity retention but also changes in adsorbent structure and kinetics, to truly advance the development of viable adsorbents for industrial CO2 capture.
Beyond the pursuit of high adsorption capacity and selectivity, the practical deployment of porous carbon adsorbents ultimately hinges on their overall process economics. The cost of adsorbent production is heavily influenced by the choice of precursor (e.g., low-cost biomass vs. refined chemicals) and the activation method (e.g., corrosive chemical activation vs. greener physical activation). More critically, the energy required for regeneration—often the dominant operational expense—is directly linked to the adsorption mechanism: physisorption-dominated carbons enable low-temperature (e.g., 80–120 °C) or vacuum-driven regeneration, whereas materials enhanced by strong chemisorption (e.g., metal-impregnated carbons) typically demand higher energy input for desorption. Furthermore, the cyclic stability and lifetime of the adsorbent under real flue gas conditions directly amortize its initial cost. Therefore, future research on enhancing CO2 adsorption must increasingly couple material design with preliminary techno-economic considerations, evaluating not just the “grams of CO2 captured per gram of adsorbent” but also the “kilojoules required per mole of CO2 regenerated” and the “cost per ton of CO2 captured over the adsorbent’s lifetime.
5. Conclusions and Future Perspectives
Porous carbons exhibit considerable advantages in CO2 capture; however, their insufficient adsorption capacity and poor selectivity hinder practical large-scale applications. To address these limitations, numerous modification strategies have been developed. This review systematically summarizes the research progress in porous carbon-based CO2 adsorption, with a particular focus on strategies for enhancing adsorption performance. Key strategies are comprehensively discussed, including porosity regulation via various methods, surface modification (e.g., heteroatom doping, metal impregnation), and other emerging approaches. Despite significant advancements in this field, further studies are required to improve the adsorption and separation performance of porous carbons, focusing on the following aspects:
- (1)
-
Developing efficient pore-formation strategies to fabricate porous carbons with tailored micro-meso-macro porous structures remains highly desirable yet challenging. Traditional physical or chemical activation alone typically yields microporous or micro-mesoporous carbons. Future research should prioritize establishing the relationship between the micro-meso-macro pore ratio and CO2 mass transfer/adsorption capacity. As highlighted earlier, adsorption capacity is predominantly determined by micropores—especially ultramicropores (<0.8 nm)—whereas mesopores and macropores govern CO2 diffusion, adsorption rate, and regenerability. Thus, fabricating porous carbons with optimized micro-meso-macro structures and elucidating the effect of pore ratio on CO2 capture performance is of great significance.
- (2)
-
Greater efforts should be devoted to developing synthetic strategies for heteroatom-doped porous carbons with selective introduction of functional groups that enhance CO2 adsorption and separation. Heteroatom-derived functionalities exert distinct effects on CO2 adsorption: for nitrogen doping, only pyridonic and pyridinic N exhibit positive effects, whereas quaternary and oxidized N have negligible or even adverse impacts. Unfortunately, effective methods for doping specific nitrogen-containing groups are lacking, and precise regulation of their distribution, content, and thermal stability remains challenging. Simultaneously, the relationship between nitrogen doping and CO2 adsorption performance needs to be clarified from both fundamental and application perspectives. Additionally, more research should focus on the preparation, application, and mechanism of porous carbons doped with other heteroatoms (e.g., S, P) for CO2 adsorption.
- (3)
-
It is crucial to develop efficient strategies for fabricating porous carbons with tunable pore structures and optimal surface functionalities. Moreover, understanding the competitive adsorption between CO2 and flue gas impurities (e.g., H2O, NOx, SOx) is essential for designing high-performance adsorbents. Generally, heteroatom doping (N, S, P, etc.) often reduces specific surface area and causes pore collapse, increasing hydrophilicity while decreasing CO2 adsorption capacity. Operating conditions (temperature, pressure, coexisting gases) also significantly affect CO2 adsorption during flue gas recycling. Notably, flue gas impurities (dust, SO2, NOx) are detrimental to adsorbents and difficult to completely remove; even low concentrations accumulate to significant levels over long-term operation due to the large flow rate and prolonged duration of flue gases, severely degrading adsorption performance. Elucidating the competitive adsorption mechanism between CO2 and these pollutants is therefore critical for developing porous carbon-based adsorbents with excellent performance in real flue gas environments.
- (4)
-
Increased attention should be paid to the preparation and application of metal-impregnated porous carbons (MPCs) for CO2 adsorption. Recent studies have demonstrated that MPCs are efficient and cost-effective due to their high regenerability; however, they have not been widely adopted in practical CO2 capture. To promote their large-scale application, future advancements should focus on two aspects: developing novel hybrid MPCs and optimizing regeneration strategies. Comprehensive investigations into the potential of MPCs as alternative CO2 adsorbents are required, specifically focusing on: (I) optimizing preparation parameters (metal type, loading amount, synthesis techniques) to enhance adsorption performance; (II) selecting suitable carbon sources and fabricating porous carbons with appropriate structures for efficient metal oxide impregnation; (III) developing functionalized hybrid MPCs via compositing with other inorganic/organic nanostructures (e.g., amines, nitrogen-containing compounds); and (IV) clarifying the CO2-MPC interaction mechanism through combined experimental and theoretical studies.
Statement of the Use of Generative AI and AI-Assisted Technologies in the Writing Process
During the preparation of this manuscript, the authors used Deepseek in order to enhance linguistic quality. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
Acknowledgments
We express our thanks for funding support from the National Natural Science Foundation of China (No. 52063016, No. 52372048, No. 52202336), the Key Laboratory of Advanced Materials of Ministry of Education (No. Advmat-2421), and the Graduate Innovative Fund of Wuhan Institute of Technology (No. CX2024037).
Author Contributions
Writing—review & editing, Funding, J.W.; Resources, G.Y.; Validation, Investigation, Formal analysis, Funding, L.W.; Investigation, Formal analysis, Funding, B.Z.; Formal analysis, Z.W.; Supervision, Project administration, Z.H.; Investigation, Formal analysis, G.L.; Writing—review & editing, Project administration, Conceptualization, M.W.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Relevant information and dates can be made available upon request.
Funding
The work was financially supported by the Fund of National Natural Science Foundation of China (No. 52063016, No. 52372048, No. 52202336), the Fund of Key Laboratory of Advanced Materials of Ministry of Education (No. Advmat-2421), and the Graduate Innovative Fund of Wuhan Institute of Technology (No. CX2025146).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
Ghanbari T, Abnisa F, Daud WMAW. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. DOI:10.1016/j.scitotenv.2019.135090 [Google Scholar]
-
Rong W, Ding M, Wang Y, Kong S, Yao J. Porous biochar with a tubular structure for photothermal CO2 cycloaddition: One-step doping versus two-step doping. Sep. Purif. Technol. 2025, 353, 128427. DOI:10.1016/j.seppur.2024.128427 [Google Scholar]
-
Lee G, Ahmed I, Jhung SH. CO2 adsorption using functionalized metal-organic frameworks under low pressure: Contribution of functional groups, excluding amines, to adsorption. Chem. Eng. J. 2024, 481, 148440. DOI:10.1016/j.cej.2023.148440 [Google Scholar]
-
Adegoke KA, Oyedotun KO, Ighalo JO, Amaku JF, Olisah C, Adeola AO, et al. Cellulose derivatives and cellulose-metal-organic frameworks for CO2 adsorption and separation. J. CO2 Util. 2022, 64, 102163. DOI:10.1016/j.jcou.2022.102163 [Google Scholar]
-
Peters GP, Le Quéré C, Andrew RM, Canadell JG, Friedlingstein P, Ilyina T, et al. Towards real-time verification of CO2 emissions. Nat. Clim. Change. 2017, 7, 848–850. DOI:10.1038/s41558-017-0013-9 [Google Scholar]
-
Friedlingstein P, O’sullivan M, Jones MW, Andrew RM, Gregor L, Hauck J, et al. Global carbon budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. DOI:10.5194/essd-14-4811-2022 [Google Scholar]
-
Rogelj J, Den Elzen M, Höhne N, Fransen T, Fekete H, Winkler H, et al. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. DOI:10.1038/nature18307 [Google Scholar]
-
Karimi M, Shirzad M, Silva JAC, Rodrigues AE. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. DOI:10.1016/j.jcou.2022.101890 [Google Scholar]
-
Keshavarz L, Ghaani MR, MacElroy JD, English NJ. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterisation. Chem. Eng. J. 2021, 412, 128604. DOI:10.1016/j.cej.2021.128604 [Google Scholar]
-
Shen Y. Preparation of renewable porous carbons for CO2 capture—A review. Fuel Process. Technol. 2022, 236, 107437. DOI:10.1016/j.fuproc.2022.107437 [Google Scholar]
-
Balasubramanian R, Chowdhury S. Recent advances and progress in the development of graphene-based adsorbents for CO2 capture. J. Mater. Chem. A 2015, 3, 21968–21989. DOI:10.1039/c5ta04822b [Google Scholar]
-
Li J-R, Ma Y, McCarthy MC, Sculley J, Yu J, Jeong H-K, et al. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. DOI:10.1016/j.ccr.2011.02.012 [Google Scholar]
-
Rochelle GT. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. DOI:10.1126/science.1176731 [Google Scholar]
-
Zhang Y, Wang S, Feng D, Gao J, Dong L, Zhao Y, et al. Functional biochar synergistic solid/liquid-phase CO2 capture: A review. Energy Fuels 2022, 36, 2945–2970. DOI:10.1021/acs.energyfuels.1c04372 [Google Scholar]
-
Fazlollahi F, Saeidi S, Safdari MS, Sarkari M, Klemeš JJ, Baxter LL. Effect of operating conditions on cryogenic carbon dioxide removal. Energy Technol. 2017, 5, 1588–1598. DOI:10.1002/ente.201600802 [Google Scholar]
-
Creamer AE, Gao B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. DOI:10.1021/acs.est.6b00627 [Google Scholar]
-
Karimi M, Rodrigues AE, Silva JA. Designing a simple volumetric apparatus for measuring gas adsorption equilibria and kinetics of sorption. Application and validation for CO2, CH4 and N2 adsorption in binder-free beads of 4A zeolite. Chem. Eng. J. 2021, 425, 130538. DOI:10.1016/j.cej.2021.130538 [Google Scholar]
-
Zhu X, Li S, Shi Y, Cai N. Recent advances in elevated-temperature pressure swing adsorption for carbon capture and hydrogen production. Prog. Energy Combust. Sci. 2019, 75, 100784. DOI:10.1016/j.pecs.2019.100784 [Google Scholar]
-
Sreenivasulu B, Sreedhar I, Suresh P, Raghavan KV. Development trends in porous adsorbents for carbon capture. Environ. Sci. Technol. 2015, 49, 12641–12661. DOI:10.1021/acs.est.5b03149 [Google Scholar]
-
Omodolor IS, Otor HO, Andonegui JA, Allen BJ, Alba-Rubio AC. Dual-function materials for CO2 capture and conversion: A review. Ind. Eng. Chem. Res. 2020, 59, 17612–17631. DOI:10.1021/acs.iecr.0c02218 [Google Scholar]
-
Belaissaoui B, Le Moullec Y, Willson D, Favre E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Membr. Sci. 2012, 415, 424–434. DOI:10.1016/j.memsci.2012.05.029 [Google Scholar]
-
Sun J, Liang C, Tong X, Guo Y, Li W, Zhao C, et al. Evaluation of high-temperature CO2 capture performance of cellulose-templated CaO-based pellets. Fuel 2019, 239, 1046–1054. DOI:10.1016/j.fuel.2018.11.123 [Google Scholar]
-
Veneman R, Zhao W, Li Z, Cai N, Brilman DW. Adsorption of CO2 and H2O on supported amine sorbents. Int. J. Greenh. Gas Control. 2014, 41, 268–275. DOI:10.1016/j.ijggc.2015.07.014 [Google Scholar]
-
Karimi M, Silva JAC, Gonçalves CNDP, de Tuesta JLD, Rodrigues AE, Gomes HT. CO2 capture in chemically and thermally modified activated carbons using breakthrough measurements: experimental and modeling study. Ind. Eng. Chem. Res. 2018, 57, 11154–11166. DOI:10.1021/acs.iecr.8b00953 [Google Scholar]
-
Obi D, Onyekuru S, Orga A. Review of recent process developments in the field of carbon dioxide (CO2) capture from power plants flue gases and the future perspectives. Int. J. Sustain. Energy 2024, 43, 2317137. DOI:10.1080/14786451.2024.2317137 [Google Scholar]
-
Li Z, Shi K, Zhai L, Wang Z, Wang H, Zhao Y, et al. Constructing multiple sites of metal-organic frameworks for efficient adsorption and selective separation of CO2. Sep. Purif. Technol. 2023, 307, 122725. DOI:10.1016/j.seppur.2022.122725 [Google Scholar]
-
Essih S, Vilarrasa-Garcia E, Azevedo DCS, Ballesteros-Plata D, Barroso-Martin I, Infantes-Molina A, et al. Zeolites synthesis from phyllosilicates and their performance for CO2 adsorption. Environ. Sci. Pollut. Res. 2024, 31, 37298–37315. DOI:10.1007/s11356-024-33685-0 [Google Scholar]
-
Guo S, Li Y, Wang Y, Wang L, Sun Y, Liu L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100059. DOI:10.1016/j.ccst.2022.100059 [Google Scholar]
-
Creamer AE, Gao B, Zhang M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. DOI:10.1016/j.cej.2014.03.105 [Google Scholar]
-
Wu R, Bao A. Preparation of cellulose carbon material from cow dung and its CO2 adsorption performance. J. CO2 Util. 2023, 68, 102377. DOI:10.1016/j.jcou.2022.102377 [Google Scholar]
-
Hou Y, Chen Y, He X, Wang F, Cai Q, Shen B. Insights into the adsorption of CO2, SO2 and NOx in flue gas by carbon materials: A critical review. Chem. Eng. J. 2024, 490, 151424. DOI:10.1016/j.cej.2024.151424 [Google Scholar]
-
Di H, Zhang T, Ma H, Zhang G, Li K, Han J, et al. Biomass tar modified biochar with enhanced micropores and oxygen content for high efficiency CO2 capture. Sep. Purif. Technol. 2025, 378, 134678. DOI:10.1016/j.seppur.2025.134678 [Google Scholar]
-
Raganati F, Miccio F, Ammendola P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. DOI:10.1021/acs.energyfuels.1c01618 [Google Scholar]
-
Alfe M, Policicchio A, Lisi L, Gargiulo V. Solid sorbents for CO2 and CH4 adsorption: The effect of metal organic framework hybridization with graphene-like layers on the gas sorption capacities at high pressure. Renew. Sustain. Energy Rev. 2021, 141, 110816. DOI:10.1016/j.rser.2021.110816 [Google Scholar]
-
Qiu T, Cao W, Xie K, Ahmad F, Zhao W, Mostafa E, et al. CO2 capture performances of H3PO4/KOH activated microwave pyrolyzed porous biochar. Sustain. Carbon Mater. 2025, 1, e004. DOI:10.48130/scm-0025-0004 [Google Scholar]
-
Sun J, Li H, Liu Q, Zhong W, Dong W. Recent advances in biomass-derived porous carbons for post-combustion CO2 capture: From preparation and adsorption mechanisms to applications. Chem. Eng. J. 2025, 520, 165890. DOI:10.1016/j.cej.2025.165890 [Google Scholar]
-
Harris PJF. Structural models of non-graphitising carbon: A brief history. C 2025, 11, 78. DOI:10.3390/c11040078 [Google Scholar]
-
Whiffen DH. Manual of Symbols and Terminology for Physicochemical Quantities and Units; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
-
Yang RT. Adsorbents: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
-
Zhang J, Li Q, Zhang J, Liu H, Wang H, Zhang J. Enhanced CO2 absorption in amine-based carbon capture aided by coconut shell-derived nitrogen-doped biochar. Sep. Purif. Technol. 2025, 353, 128451. DOI:10.1016/j.seppur.2024.128451 [Google Scholar]
-
Sakib S, Woo HK, Karua P, Salauddin M, Zheng W, Cai L, et al. Sustainable production of nitrogen-doped hierarchical porous carbons from fruit peel wastes for high-performance CO2 capture. Sep. Purif. Technol. 2026, 380, 135501. DOI:10.1016/j.seppur.2025.135501 [Google Scholar]
-
He S, Chen G, Xiao H, Shi G, Ruan C, Ma Y, et al. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 2021, 582, 90–101. DOI:10.1016/j.jcis.2020.08.021 [Google Scholar]
-
Wei H, Deng S, Hu B, Chen Z, Wang B, Huang J, et al. Granular bamboo-derived activated carbon for high CO2 adsorption: the dominant role of narrow micropores. ChemSusChem 2012, 5, 2354–2360. DOI:10.1002/cssc.201200570 [Google Scholar]
-
Silvestre-Albero J, Wahby A, Sepúlveda-Escribano A, Martínez-Escandell M, Kaneko K, Rodríguez-Reinoso F. Ultrahigh CO2 adsorption capacity on carbon molecular sieves at room temperature. Chem. Commun. 2011, 47, 6840–6842. DOI:10.1039/c1cc11618e [Google Scholar]
-
Lu T, Bai J, Demir M, Hu X, Huang J, Wang L. Synthesis of potassium bitartrate-derived porous carbon via a facile and self-activating strategy for CO2 adsorption application. Sep. Purif. Technol. 2022, 296, 121368. DOI:10.1016/j.seppur.2022.121368 [Google Scholar]
-
Nazir G, Rehman A, Park S-J. Self-activated, urea modified microporous carbon cryogels for high-performance CO2 capture and separation. Carbon 2022, 192, 14–29. DOI:10.1016/j.carbon.2022.02.040 [Google Scholar]
-
Xing L-A, Yang F, Zhong X, Liu Y, Lu H, Guo Z, et al. Ultra-microporous cotton fiber-derived activated carbon by a facile one-step chemical activation strategy for efficient CO2 adsorption. Sep. Purif. Technol. 2023, 324, 124470. DOI:10.1016/j.seppur.2023.124470 [Google Scholar]
-
Kwiatkowski M, Kalderis D, Tono W, Tsubota T. Numerical analysis of the micropore structure of activated carbons focusing on optimum CO2 adsorption. J. CO2 Util. 2022, 60, 101996. DOI:10.1016/j.jcou.2022.101996 [Google Scholar]
-
Singh G, Lakhi KS, Sil S, Bhosale SV, Kim I, Albahily K, et al. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. DOI:10.1016/j.carbon.2019.03.050 [Google Scholar]
-
Chao C, Deng Y, Dewil R, Baeyens J, Fan X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. DOI:10.1016/j.rser.2020.110490 [Google Scholar]
-
Zhai J, Wang Z, Ouyang J, Xue B, Liu C, Xiao R. Glucose-driven synthesis of N-doped porous carbon from sludge for CO2 removal in biomass syngas. Chem. Eng. J. 2025, 512, 162722. DOI:10.1016/j.cej.2025.162722 [Google Scholar]
-
Zeng L, Zeng S, Liu P, Li H, Chen W, Li K. Unveiling the role of pentagonal topological defects in lignocellulose-derived self-assembled N-O co-doped micro-mesoporous biochar for enhanced CO2 adsorption. Carbon Capture Sci. Technol. 2025, 15, 100404. DOI:10.1016/j.ccst.2025.100404 [Google Scholar]
-
Tang Z, Zhu J, Zhao Z, Dong H, Du Q, Feng D, et al. Synergistic enhancement of CO2 adsorption performance of porous biochar by N/O heteroatoms and pore structure: experiment and DFT simulation. J. Environ. Chem. Eng. 2025, 13, 120149. DOI:10.1016/j.jece.2025.120149 [Google Scholar]
-
Yin Q, Gao Y, Wang R, Zhu X, Gao X, Zhao Z, et al. Influence of Fe-N co-doping on biochar on the adsorption of CO2. Chem. Eng. Sci. 2026, 320, 122702. DOI:10.1016/j.ces.2025.122702 [Google Scholar]
-
Barber PS, Griggs CS, Gurau G, Liu Z, Li S, Li Z, et al. Coagulation of chitin and cellulose from 1-ethyl-3-methylimidazolium acetate ionic-liquid solutions using carbon dioxide. Angew. Chem. Int. Ed. 2013, 52, 12350–12353. DOI:10.1002/ange.201304604 [Google Scholar]
-
Shao J, Wang Y, Che M, Xiao Q, Demir M, Al Mesfer MK, et al. N, S Co-doped porous carbons from coconut shell for selective CO2 adsorption. J. Energy Inst. 2025, 123, 102273. DOI:10.1016/j.joei.2025.102273 [Google Scholar]
-
Kolle JM, Fayaz M, Sayari A. Understanding the effect of water on CO2 adsorption. Chem. Rev. 2021, 121, 7280–7345. DOI:10.1021/acs.chemrev.0c00762 [Google Scholar]
-
Li YY, Han KK, Lin WG, Wan MM, Wang Y, Zhu JH. Fabrication of a new MgO/C sorbent for CO2 capture at elevated temperature. J. Mater. Chem. A 2013, 1, 12919–12925. DOI:10.1039/c3ta12261a [Google Scholar]
-
Zhang H, Goeppert A, Olah GA, Prakash GS. Remarkable effect of moisture on the CO2 adsorption of nano-silica supported linear and branched polyethylenimine. J. CO2 Util. 2017, 19, 91–99. DOI:10.1016/j.jcou.2017.03.008 [Google Scholar]
-
Guo B, Wang Y, Shen X, Qiao X, Jia L, Xiang J, et al. Study on CO2 capture characteristics and kinetics of modified potassium-based adsorbents. Materials 2020, 13, 877. DOI:10.3390/ma13040877 [Google Scholar]
-
Akpasi SO, Isa YM. Effect of operating variables on CO2 adsorption capacity of activated carbon, kaolinite, and activated carbon–Kaolinite composite adsorbent. Water-Energy Nexus 2022, 5, 21–28. DOI:10.1016/j.wen.2022.08.001 [Google Scholar]
-
Luo J, Liu B, Shi R, Guo Y, Feng Q, Liu Z, et al. The effects of nitrogen functional groups and narrow micropore sizes on CO2 adsorption onto N-doped biomass-based porous carbon under different pressure. Microporous Mesoporous Mater. 2021, 327, 111404. DOI:10.1016/j.micromeso.2021.111404 [Google Scholar]
-
Wang Q, Tay HH, Zhong Z, Luo J, Borgna A. Synthesis of high-temperature CO2 adsorbents from organo-layered double hydroxides with markedly improved CO2 capture capacity. Energy Environ. Sci. 2012, 5, 7526–7530. DOI:10.1039/c2ee21409a [Google Scholar]
-
Gao Y, Zhang Z, Wu J, Yi X, Zheng A, Umar A, et al. Comprehensive investigation of CO2 adsorption on Mg–Al–CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782–12790. DOI:10.1039/c3ta13039h [Google Scholar]
-
Ramírez-Moreno MJ, Romero-Ibarra IC, Hernández-Pérez M, Pfeiffer H. CO2 adsorption at elevated pressure and temperature on Mg–Al layered double hydroxide. Ind. Eng. Chem. Res. 2014, 53, 8087–8094. DOI:10.1021/ie5010515 [Google Scholar]
-
Kong Y, Shen X, Fan M, Yang M, Cui S. Dynamic capture of low-concentration CO2 on amine hybrid silsesquioxane aerogel. Chem. Eng. J. 2016, 283, 1059–1068. DOI:10.1016/j.cej.2015.08.034 [Google Scholar]
-
Baraka F, Labidi J. The emergence of nanocellulose aerogels in CO2 adsorption. Sci. Total Environ. 2023, 912, 169093. DOI:10.1016/j.scitotenv.2023.169093 [Google Scholar]
-
Siegelman RL, Milner PJ, Kim EJ, Weston SC, Long JR. Challenges and opportunities for adsorption-based CO2 capture from natural gas combined cycle emissions. Energy Environ. Sci. 2019, 12, 2161–2173. DOI:10.1039/c9ee00505f [Google Scholar]
-
Long Y, Tian H, Lee C-H, Li H, Zeng Z, Yang Z, et al. Competitive adsorption of H2O and CO2 on nitrogen-doped biochar with rich-oxygen functional groups. Sep. Purif. Technol. 2025, 359, 130476. DOI:10.1016/j.seppur.2024.130476 [Google Scholar]
-
Li G, Xiao P, Zhang J, Webley PA, Xu D. The role of water on postcombustion CO2 capture by vacuum swing adsorption: Bed layering and purge to feed ratio. AlChE J. 2014, 60, 673–689. DOI:10.1002/aic.14281 [Google Scholar]
-
Zhang J, Xiao P, Li G, Webley PA. Effect of flue gas impurities on CO2 capture performance from flue gas at coal-fired power stations by vacuum swing adsorption. Energy Procedia. 2009, 1, 1115–1122. DOI:10.1016/j.egypro.2009.01.147 [Google Scholar]
-
Plaza MG, González AS, Rubiera F, Pevida C. Water vapour adsorption by a coffee-based microporous carbon: Effect on CO2 capture. J. Chem. Technol. Biotechnol. 2015, 90, 1592–1600. DOI:10.1002/jctb.4636 [Google Scholar]
-
Xu D, Xiao P, Zhang J, Li G, Xiao G, Webley PA, et al. Effects of water vapour on CO2 capture with vacuum swing adsorption using activated carbon. Chem. Eng. J. 2013, 230, 64–72. DOI:10.1016/j.cej.2013.06.080 [Google Scholar]
-
Wahby A, Silvestre-Albero J, Sepúlveda-Escribano A, Rodríguez-Reinoso F. CO2 adsorption on carbon molecular sieves. Microporous Mesoporous Mater. 2012, 164, 280–287. DOI:10.1016/j.micromeso.2012.06.034 [Google Scholar]
-
Cossarutto L, Zimny T, Kaczmarczyk J, Siemieniewska T, Bimer J, Weber J. Transport and sorption of water vapour in activated carbons. Carbon 2001, 39, 2339–2346. DOI:10.1016/s0008-6223(01)00065-3 [Google Scholar]
-
Keller JU, Staudt R. Gas Adsorption Equilibria: Experimental Methods and Adsorptive Isotherms; Springer: New York, NY, USA, 2005. [Google Scholar]
-
Do D, Do H. A model for water adsorption in activated carbon. Carbon 2000, 38, 767–773. DOI:10.1016/S0008-6223(99)00159-1 [Google Scholar]
-
Dubinin M. Water vapor adsorption and the microporous structures of carbonaceous adsorbents. Carbon 1980, 18, 355–364. DOI:10.1016/0008-6223(80)90007-X [Google Scholar]
-
Barton SS, Evans MJ, MacDonald JA. The adsorption of water vapor by porous carbon. Carbon 1991, 29, 1099–1105. DOI:10.1016/0008-6223(91)90026-F [Google Scholar]
-
Müller EA, Rull LF, Vega LF, Gubbins KE. Adsorption of water on activated carbons: a molecular simulation study. J. Phys. Chem. 1996, 100, 1189–1196. DOI:10.1021/jp952233w [Google Scholar]
-
Durán I, Rubiera F, Pevida C. Separation of CO2 in a solid waste management incineration facility using activated carbon derived from pine sawdust. Energies 2017, 10, 827. DOI:10.3390/en10060827 [Google Scholar]
-
Younas M, Leong LK, Mohamed AR, Sethupathi S. CO2 adsorption by modified palm shell activated carbon (PSAC) via chemical and physical activation and metal impregnation. Chem. Eng. Commun. 2016, 203, 1455–1463. DOI:10.1080/00986445.2016.1201660 [Google Scholar]
-
Gray M, Johnson MG, Dragila MI, Kleber M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. DOI:10.1016/j.biombioe.2013.12.010 [Google Scholar]
-
Kinney T, Masiello C, Dugan B, Hockaday W, Dean M, Zygourakis K, et al. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. DOI:10.1016/j.biombioe.2012.01.033 [Google Scholar]
-
Kameyama K, Miyamoto T, Iwata Y. The preliminary study of water-retention related properties of biochar produced from various feedstock at different pyrolysis temperatures. Materials 2019, 12, 1732. DOI:10.3390/ma12111732 [Google Scholar]
-
Ahmad M, Lee SS, Dou X, Mohan D, Sung J-K, Yang JE, et al. Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. DOI:10.1016/j.biortech.2012.05.042 [Google Scholar]
-
Fang Q, Chen B, Lin Y, Guan Y. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol. 2014, 48, 279–288. DOI:10.1021/es403711y [Google Scholar]
-
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. DOI:10.1016/j.chemosphere.2013.10.071 [Google Scholar]
-
Sun Y, Wang Y, Zhang Y, Zhou Y, Zhou L. CO2 sorption in activated carbon in the presence of water. Chem. Phys. Lett. 2007, 437, 14–16. DOI:10.1016/j.cplett.2007.02.008 [Google Scholar]
-
Shi S, Ochedi FO, Yu J, Liu Y. Porous biochars derived from microalgae pyrolysis for CO2 adsorption. Energy Fuels 2021, 35, 7646–7656. DOI:10.1021/acs.energyfuels.0c04091 [Google Scholar]
-
Igalavithana AD, Choi SW, Shang J, Hanif A, Dissanayake PD, Tsang DC, et al. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci. Total Environ. 2020, 739, 139845. DOI:10.1016/j.scitotenv.2020.139845 [Google Scholar]
-
Liu S-H, Huang Y-Y. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Cleaner Prod. 2018, 175, 354–360. DOI:10.1016/j.jclepro.2017.12.076 [Google Scholar]
-
Tefera DT, Hashisho Z, Philips JH, Anderson JE, Nichols M. Modeling competitive adsorption of mixtures of volatile organic compounds in a fixed-bed of beaded activated carbon. Environ. Sci. Technol. 2014, 48, 5108–5117. DOI:10.1021/es404667f [Google Scholar]
-
Hu J, Liu Y, Liu J, Gu C. Effects of water vapor and trace gas impurities in flue gas on CO2 capture in zeolitic imidazolate frameworks: The significant role of functional groups. Fuel 2017, 200, 244–251. DOI:10.1016/j.fuel.2017.03.079 [Google Scholar]
-
Zhang W, Rabiei S, Bagreev A, Zhuang M, Rasouli F. Study of NO adsorption on activated carbons. Appl. Catal. B 2008, 83, 63–71. DOI:10.1016/j.apcatb.2008.02.003 [Google Scholar]
-
Chen J, Cao F, Chen S, Ni M, Gao X, Cen K. Adsorption kinetics of NO on ordered mesoporous carbon (OMC) and cerium-containing OMC (Ce-OMC). Appl. Surf. Sci. 2014, 317, 26–34. DOI:10.1016/j.apsusc.2014.08.067 [Google Scholar]
-
Sun F, Gao J, Zhu Y, Chen G, Wu S, Qin Y. Adsorption of SO2 by typical carbonaceous material: A comparative study of carbon nanotubes and activated carbons. Adsorption 2013, 19, 959–966. DOI:10.1007/s10450-013-9504-9 [Google Scholar]
-
Sethupathi S, Zhang M, Rajapaksha AU, Lee SR, Mohamad Nor N, Mohamed AR, et al. Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. DOI:10.3390/su9010121 [Google Scholar]
-
Zhao W, Guo X, Zhou Z, Wang Z, Han M, Wang X. Preparation and CO2 adsorption of N-doped biochar and effect of acetone on its adsorption performance. J. Environ. Manag. 2025, 389, 126041. DOI:10.1016/j.jenvman.2025.126041 [Google Scholar]
-
Wang Y, Hu X, Hao J, Ma R, Guo Q, Gao H, et al. Nitrogen and oxygen codoped porous carbon with superior CO2 adsorption performance: A combined experimental and DFT calculation study. Ind. Eng. Chem. Res. 2019, 58, 13390–13400. DOI:10.1021/acs.iecr.9b01454 [Google Scholar]
-
Yi H, Zuo Y, Liu H, Tang X, Zhao S, Wang Z, et al. Simultaneous removal of SO2, NO, and CO2 on metal-modified coconut shell activated carbon. Water Air Soil Pollut. 2014, 225, 1965. DOI:10.1007/s11270-014-1965-2 [Google Scholar]
-
Deng S, Wei H, Chen T, Wang B, Huang J, Yu G. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem. Eng. J. 2014, 253, 46–54. DOI:10.1016/j.cej.2014.04.115 [Google Scholar]
-
Abbaspour N, Jordan C, Tondl G, Wąsik P, Gholizadeh T, Tomasetig D, et al. Activated biochars from heavy metal-contaminated biomass for CO2 capture: Adsorption performance and dominant mechanisms. J. CO2 Util. 2025, 101, 103217. DOI:10.1016/j.jcou.2025.103217 [Google Scholar]
-
Rashidi NA, Yusup S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. DOI:10.1016/j.jcou.2015.11.002 [Google Scholar]
-
Jagiello J, Kenvin J, Celzard A, Fierro V. Enhanced resolution of ultra micropore size determination of biochars and activated carbons by dual gas analysis using N2 and CO2 with 2D-NLDFT adsorption models. Carbon 2019, 144, 206–215. DOI:10.1016/j.carbon.2018.12.028 [Google Scholar]
-
Sun Y, Zhao J, Wang J, Tang N, Zhao R, Zhang D, et al. Sulfur-doped millimeter-sized microporous activated carbon spheres derived from sulfonated poly (styrene–divinylbenzene) for CO2 capture. J. Phys. Chem. C 2017, 121, 10000–10009. DOI:10.1021/acs.jpcc.7b02195 [Google Scholar]
-
Singh G, Lee J, Karakoti A, Bahadur R, Yi J, Zhao D, et al. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. DOI:10.1039/d0cs00075b [Google Scholar]
-
Goetz V, Pupier O, Guillot A. Carbon dioxide-methane mixture adsorption on activated carbon. Adsorption 2006, 12, 55–63. DOI:10.1007/s10450-006-0138-z [Google Scholar]
-
Hu Z, Lei L, Li Y, Ni Y. Chromium adsorption on high-performance activated carbons from aqueous solution. Sep. Purif. Technol. 2003, 31, 13–18. DOI:10.1016/s1383-5866(02)00149-1 [Google Scholar]
-
Xie T, He J, Xu L-C, Yan T, Pan Q-W, Wang L-W, et al. Preparation of N-doped porous biochar with high CO2 adsorption performance via one-step molten salt thermal treatment. Chem. Eng. J. 2025, 521, 166621. DOI:10.1016/j.cej.2025.166621 [Google Scholar]
-
Deng S, Hu B, Chen T, Wang B, Huang J, Wang Y, et al. Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 2015, 21, 125–133. DOI:10.1007/s10450-015-9655-y [Google Scholar]
-
Nandi M, Okada K, Dutta A, Bhaumik A, Maruyama J, Derks D, et al. Unprecedented CO2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation. Chem. Commun. 2012, 48, 10283–10285. DOI:10.1039/c2cc35334b [Google Scholar]
-
Sevilla M, Fuertes AB. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. DOI:10.1039/c0ee00784f [Google Scholar]
-
Coromina HM, Walsh DA, Mokaya R. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications. J. Mater. Chem. A 2016, 4, 280–289. DOI:10.1039/c5ta09202g [Google Scholar]
-
Liu C, Wang J, Zhang S, Wei C, Cao L, Zhou Y, et al. Ultramicropore-rich N-doped porous biochar from discarded cigarette butts for efficient CO2 capture with ultra-high adsorption capacity and selectivity. Sep. Purif. Technol. 2025, 358, 130205. DOI:10.1016/j.seppur.2024.130205 [Google Scholar]
-
Li J, Michalkiewicz B, Min J, Ma C, Chen X, Gong J, et al. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem. Eng. J. 2019, 360, 250–259. DOI:10.1016/j.cej.2018.11.204 [Google Scholar]
-
Everett DH, Powl JC. Adsorption in slit-like and cylindrical micropores in the henry’s law region. A model for the microporosity of carbons. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1976, 72, 619–636. DOI:10.1039/f19767200619 [Google Scholar]
-
Li D, Zhou J, Wang Y, Tian Y, Wei L, Zhang Z, et al. Effects of activation temperature on densities and volumetric CO2 adsorption performance of alkali-activated carbons. Fuel 2019, 238, 232–239. DOI:10.1016/j.fuel.2018.10.122 [Google Scholar]
-
Liu J, Liu X, Sun Y, Sun C, Liu H, Stevens LA, et al. High density and super ultra-microporous-activated carbon macrospheres with high volumetric capacity for CO2 capture. Adv. Sustain. Syst. 2018, 2, 1700115. DOI:10.1002/adsu.201700115 [Google Scholar]
-
Haffner-Staton E, Balahmar N, Mokaya R. High yield and high packing density porous carbon for unprecedented CO2 capture from the first attempt at activation of air-carbonized biomass. J. Mater. Chem. A 2016, 4, 13324–13335. DOI:10.1039/c6ta06407h [Google Scholar]
-
Guo L, Yang J, Hu G, Hu X, Wang L, Dong Y, et al. Role of hydrogen peroxide preoxidizing on CO2 adsorption of nitrogen-doped carbons produced from coconut shell. ACS Sustain. Chem. Eng. 2016, 4, 2806–2813. DOI:10.1021/acssuschemeng.6b00327 [Google Scholar]
-
Fan Z, Cheng Z, Feng J, Xie Z, Liu Y, Wang Y. Ultrahigh volumetric performance of a free-standing compact N-doped holey graphene/PANI slice for supercapacitors. J. Mater. Chem. A 2017, 5, 16689–16701. DOI:10.1039/c7ta04384h [Google Scholar]
-
Qie Z, Wang L, Sun F, Xiang H, Wang H, Gao J, et al. Tuning porosity of coal-derived activated carbons for CO2 adsorption. Front. Chem. Sci. Eng. 2022, 16, 1345–1354. DOI:10.1007/s11705-022-2155-1 [Google Scholar]
-
Ahmadpour A, Do D. The preparation of active carbons from coal by chemical and physical activation. Carbon 1996, 34, 471–479. DOI:10.1016/0008-6223(95)00204-9 [Google Scholar]
-
Rehman A, Park S-J. Comparative study of activation methods to design nitrogen-doped ultra-microporous carbons as efficient contenders for CO2 capture. Chem. Eng. J. 2018, 352, 539–548. DOI:10.1016/j.cej.2018.07.046 [Google Scholar]
-
Li D, Yang J, Zhao Y, Yuan H, Chen Y. Ultra-highly porous carbon from wasted soybean residue with tailored porosity and doped structure as renewable multi-purpose absorbent for efficient CO2, toluene and water vapor capture. J. Cleaner Prod. 2022, 337, 130283. DOI:10.1016/j.jclepro.2021.130283 [Google Scholar]
-
Chen J, Lin J, Luo J, Tian Z, Zhang J, Sun S, et al. Enhanced CO2 capture performance of N, S co-doped biochar prepared by microwave pyrolysis: Synergistic modulation of microporous structure and functional groups. Fuel 2025, 379, 132987. DOI:10.1016/j.fuel.2024.132987 [Google Scholar]
-
Wang L, Sun F, Hao F, Qu Z, Gao J, Liu M, et al. A green trace K2CO3 induced catalytic activation strategy for developing coal-converted activated carbon as advanced candidate for CO2 adsorption and supercapacitors. Chem. Eng. J. 2020, 383, 123205. DOI:10.1016/j.cej.2019.123205 [Google Scholar]
-
Yuan H, Chen J, Li D, Chen H, Chen Y. 5 Ultramicropore-rich renewable porous carbon from biomass tar with excellent adsorption capacity and selectivity for CO2 capture. Chem. Eng. J. 2019, 373, 171–178. DOI:10.1016/j.cej.2019.04.206 [Google Scholar]
-
Saha D, Kienbaum MJ. Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater. 2019, 287, 29–55. DOI:10.1016/j.micromeso.2019.05.051 [Google Scholar]
-
Saha D, Van Bramer SE, Orkoulas G, Ho H-C, Chen J, Henley DK. CO2 capture in lignin-derived and nitrogen-doped hierarchical porous carbons. Carbon 2017, 121, 257–266. DOI:10.1016/j.carbon.2017.05.088 [Google Scholar]
-
Sreńscek-Nazzal J, Kiełbasa K. Advances in modification of commercial activated carbon for enhancement of CO2 capture. Appl. Surf. Sci. 2019, 494, 137–151. DOI:10.1016/j.apsusc.2019.07.108 [Google Scholar]
-
Yong Z, Mata V, Rodrigues AE. Adsorption of carbon dioxide at high temperature—A review. Sep. Purif. Technol. 2002, 26, 195–205. DOI:10.1016/s1383-5866(01)00165-4 [Google Scholar]
-
Sun S, Yin Z, Cong B, Hong W, Zhou X, Wang Y, et al. Crystalline carbon modified hierarchical porous iron and nitrogen co-doped carbon for efficient electrocatalytic oxygen reduction. J. Colloid Interface Sci. 2021, 594, 864–873. DOI:10.1016/j.jcis.2021.03.068 [Google Scholar]
-
Wang J, Yin Y, Liu X, Liu Y, Xiao Q, Zhao L, et al. Potassium metaborate-activated boron-doped porous carbons for selective CO2 adsorption. Sep. Purif. Technol. 2025, 376, 134079. DOI:10.1016/j.seppur.2025.134079 [Google Scholar]
-
Ma X, Li L, Zeng Z, Chen R, Wang C, Zhou K, et al. Experimental and theoretical demonstration of the relative effects of O-doping and N-doping in porous carbons for CO2 capture. Appl. Surf. Sci. 2019, 481, 1139–1147. DOI:10.1016/j.apsusc.2019.03.162 [Google Scholar]
-
Li L, Wang X-F, Zhong J-J, Qian X, Song S-L, Zhang Y-G, et al. Nitrogen-enriched porous polyacrylonitrile-based carbon fibers for CO2 capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. DOI:10.1021/acs.iecr.8b01836 [Google Scholar]
-
Kumar KV, Preuss K, Lu L, Guo ZX, Titirici MM. Effect of nitrogen doping on the CO2 adsorption behavior in nanoporous carbon structures: A molecular simulation study. J. Phys. Chem. C 2015, 119, 22310–22321. DOI:10.1021/acs.jpcc.5b06017 [Google Scholar]
-
Xiao J, Yuan X, Zhang TC, Ouyang L, Yuan S. Nitrogen-doped porous carbon for excellent CO2 capture: A novel method for preparation and performance evaluation. Sep. Purif. Technol. 2022, 298, 121602. DOI:10.1016/j.seppur.2022.121602 [Google Scholar]
-
Pietrzak R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. DOI:10.1016/j.fuel.2009.04.017 [Google Scholar]
-
Nazir G, Rehman A, Ikram M, Aslam M, Zhang TC, Khalid A, et al. Facile synthesis of inherently nitrogen-doped PAN-derived microporous carbons activated with NaNH2 for selective CO2 adsorption and separation. Chem. Eng. J. 2024, 497, 154704. DOI:10.1016/j.cej.2024.154704 [Google Scholar]
-
Wang Y, Suo Y, Xu Y, Zhang Z. Enhancing CO2 adsorption performance of porous nitrogen-doped carbon materials derived from ZIFs: Insights into pore structure and surface chemistry. Sep. Purif. Technol. 2024, 335, 126117. DOI:10.1016/j.seppur.2023.126117 [Google Scholar]
-
Liu P, Qin S, Wang J, Zhang S, Tian Y, Zhang F, et al. Effective CO2 capture by in-situ nitrogen-doped nanoporous carbon derived from waste antibiotic fermentation residues. Environ. Pollut. 2023, 333, 121972. DOI:10.1016/j.envpol.2023.121972 [Google Scholar]
-
Wu W, Wu C, Liu J, Yan H, Zhang G, Li G, et al. Nitrogen-doped porous carbon through K2CO3-activated bamboo shoot shell for an efficient CO2 adsorption. Fuel 2024, 363, 130937. DOI:10.1016/j.fuel.2024.130937 [Google Scholar]
-
Zhang W, Li K, Liu B, Xu W, Sun K, Yue Y. Heteroatom-doped porous activated carbon from fir sawdust: Tunable pore structures for efficient CO2 and tetracycline adsorption. Chem. Eng. Sci. 2026, 321, 122886. DOI:10.1016/j.ces.2025.122886 [Google Scholar]
-
Montes-Morán M, Suárez D, Menéndez J, Fuente E. On the nature of basic sites on carbon surfaces: an overview. Carbon 2004, 42, 1219–1225. DOI:10.1016/j.carbon.2004.01.023 [Google Scholar]
-
Boehm HP. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. DOI:10.1016/s0008-6223(01)00165-8 [Google Scholar]
-
Voll M, Boehm H. Basische Oberflächenoxide auf Kohlenstoff—IV. Chemische Reaktionen zur Identifizierung der Oberflächengruppen. Carbon 1971, 9, 481–488. DOI:10.1016/0008-6223(71)90028-5 [Google Scholar]
-
Plaza M, Thurecht K, Pevida C, Rubiera F, Pis J, Snape C, et al. Influence of oxidation upon the CO2 capture performance of a phenolic-resin-derived carbon. Fuel Process. Technol. 2013, 110, 53–60. DOI:10.1016/j.fuproc.2013.01.011 [Google Scholar]
-
Guo Y, Tan C, Sun J, Li W, Zhang J, Zhao C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. DOI:10.1016/j.cej.2019.122736 [Google Scholar]
-
Rajapaksha AU, Chen SS, Tsang DC, Zhang M, Vithanage M, Mandal S, et al. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. DOI:10.1016/j.chemosphere.2016.01.043 [Google Scholar]
-
Budinova T, Ekinci E, Yardim F, Grimm A, Björnbom E, Minkova V, et al. Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process. Technol. 2006, 87, 899–905. DOI:10.1016/j.fuproc.2006.06.005 [Google Scholar]
-
Lahijani P, Mohammadi M, Mohamed AR. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J. CO2 Util. 2018, 26, 281–293. DOI:10.1016/j.jcou.2018.05.018 [Google Scholar]
-
Kudin KN, Ozbas B, Schniepp HC, Prud’homme RK, Aksay IA, Car R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. DOI:10.1021/nl071822y [Google Scholar]
-
Bai BC, Kim EA, Lee CW, Lee Y-S, Im JS. Effects of surface chemical properties of activated carbon fibers modified by liquid oxidation for CO2 adsorption. Appl. Surf. Sci. 2015, 353, 158–164. DOI:10.1016/j.apsusc.2015.06.046 [Google Scholar]
-
Tiwari D, Goel C, Bhunia H, Bajpai PK. Novel nanostructured carbons derived from epoxy resin and their adsorption characteristics for CO2 capture. RSC Adv. 2016, 6, 97728–97738. DOI:10.1039/c6ra18291g [Google Scholar]
-
Babu DJ, Bruns M, Schneider R, Gerthsen D, Schneider JJ. Understanding the influence of N-doping on the CO2 adsorption characteristics in carbon nanomaterials. J. Phys. Chem. C 2017, 121, 616–626. DOI:10.1021/acs.jpcc.6b11686 [Google Scholar]
-
Liu Y, Wilcox J. Molecular simulation studies of CO2 adsorption by carbon model compounds for carbon capture and sequestration applications. Environ. Sci. Technol. 2013, 47, 95–101. DOI:10.1021/es3012029 [Google Scholar]
-
Kazarian SG, Vincent MF, Bright FV, Liotta CL, Eckert CA. Specific intermolecular interaction of carbon dioxide with polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. DOI:10.1021/ja950416q [Google Scholar]
-
Fuente E, Menéndez J, Suarez D, Montes-Morán M. Basic surface oxides on carbon materials: A global view. Langmuir 2003, 19, 3505–3511. DOI:10.1021/la026778a [Google Scholar]
-
Singh G, Lakhi KS, Ramadass K, Kim S, Stockdale D, Vinu A. A combined strategy of acid-assisted polymerization and solid state activation to synthesize functionalized nanoporous activated biocarbons from biomass for CO2 capture. Microporous Mesoporous Mater. 2018, 271, 23–32. DOI:10.1016/j.micromeso.2018.05.035 [Google Scholar]
-
Liu Y, Wilcox J. Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons. Environ. Sci. Technol. 2012, 46, 1940–1947. DOI:10.1021/es204071g [Google Scholar]
-
Ma X, Li L, Chen R, Wang C, Li H, Wang S. Heteroatom-doped nanoporous carbon derived from MOF-5 for CO2 capture. Appl. Surf. Sci. 2018, 435, 494–502. DOI:10.1016/j.apsusc.2017.11.069 [Google Scholar]
-
Ren W, Saito R, Gao L, Zheng F, Wu Z, Liu B, et al. Edge phonon state of mono-and few-layer graphene nanoribbons observed by surface and interference co-enhanced raman spectroscopy. Phys. Rev. B Condens. Matter. 2010, 81, 035412. DOI:10.1103/PhysRevB.81.035412 [Google Scholar]
-
Denis PA. Concentration dependence of the band gaps of phosphorus and sulfur doped graphene. Comput. Mater. Sci. 2013, 67, 203–206. DOI:10.1016/j.commatsci.2012.08.041 [Google Scholar]
-
Su W, Yao L, Ran M, Sun Y, Liu J, Wang X. Adsorption properties of N2, CH4, and CO2 on sulfur-doped microporous carbons. J. Chem. Eng. Data 2018, 63, 2914–2920. DOI:10.1021/acs.jced.8b00227 [Google Scholar]
-
Kiciński W, Szala M, Bystrzejewski M. Sulfur-doped porous carbons: synthesis and applications. Carbon 2014, 68, 1–32. DOI:10.1016/j.carbon.2013.11.004 [Google Scholar]
-
Xia Y, Zhu Y, Tang Y. Preparation of sulfur-doped microporous carbons for the storage of hydrogen and carbon dioxide. Carbon 2012, 50, 5543–5553. DOI:10.1016/j.carbon.2012.07.044 [Google Scholar]
-
Seema H, Kemp KC, Le NH, Park S-W, Chandra V, Lee JW, et al. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon 2014, 66, 320–326. DOI:10.1016/j.carbon.2013.09.006 [Google Scholar]
-
Zhu C, Fang Q, Liu R, Dong W, Song S, Shen Y. Insights into the crucial role of electron and spin structures in heteroatom-doped covalent triazine frameworks for removing organic micropollutants. Environ. Sci. Technol. 2022, 56, 6699–6709. DOI:10.1021/acs.est.2c01781 [Google Scholar]
-
Zhou S, Wang M, Wang J, Xin H, Liu S, Wang Z, et al. Carbon phosphides: promising electric field controllable nanoporous materials for CO2 capture and separation. J. Mater. Chem. A 2020, 8, 9970–9980. DOI:10.1039/d0ta03262j [Google Scholar]
-
Khan AA, Ahmad I, Ahmad R. Influence of electric field on CO2 removal by P-doped C60-fullerene: A DFT study. Chem. Phys. Lett. 2020, 742, 137155. DOI:10.1016/j.cplett.2020.137155 [Google Scholar]
-
Luo J, Huang H, Sun S, Ma R. Delicately-controlled nitrogen species in porous biochar via microwave-coordinated phosphorus doping for enhancing CO2 capture: Active sites tuning and synergistic mechanism analysis. Sep. Purif. Technol. 2026, 382, 136032. DOI:10.1016/j.seppur.2025.136032 [Google Scholar]
-
Abuelnoor N, AlHajaj A, Khaleel M, Vega LF, Abu-Zahra MRM. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. DOI:10.1016/j.chemosphere.2021.131111 [Google Scholar]
-
Chen C-Y, Chang H-W, Kao P-C, Pan J-L, Chang J-S. Biosorption of cadmium by CO2-fixing microalga scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 105, 74–80. DOI:10.1016/j.biortech.2011.11.124 [Google Scholar]
-
Creamer AE, Gao B, Wang S. Carbon dioxide capture using various metal oxyhydroxide–biochar composites. Chem. Eng. J. 2016, 283, 826–832. DOI:10.1016/j.cej.2015.08.037 [Google Scholar]
-
Liu J, Jiang J, Meng Y, Aihemaiti A, Xu Y, Xiang H, et al. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. DOI:10.1016/j.jhazmat.2020.122026 [Google Scholar]
-
Gopalan J, Buthiyappan A, Raman AAA. Insight into metal-impregnated biomass based activated carbon for enhanced carbon dioxide adsorption: A review. J. Ind. Eng. Chem. 2022, 113, 72–95. DOI:10.1016/j.jiec.2022.06.026 [Google Scholar]
-
Ghosh S, Sarathi R, Ramaprabhu S. Magnesium oxide modified nitrogen-doped porous carbon composite as an efficient candidate for high pressure carbon dioxide capture and methane storage. J. Colloid Interface Sci. 2019, 539, 245–256. DOI:10.1016/j.jcis.2018.12.063 [Google Scholar]
-
Othman FEC, Yusof N, Samitsu S, Abdullah N, Hamid MF, Nagai K, et al. Activated carbon nanofibers incorporated metal oxides for CO2 adsorption: Effects of different type of metal oxides. J. CO2 Util. 2021, 45, 101434. DOI:10.1016/j.jcou.2021.101434 [Google Scholar]
-
Qamar S, Lei F, Liang L, Gao S, Liu K, Sun Y, et al. Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 2016, 26, 692–698. DOI:10.1016/j.nanoen.2016.06.029 [Google Scholar]
-
Khan H, Zavabeti A, Wang Y, Harrison CJ, Carey BJ, Mohiuddin M, et al. Quasi physisorptive two dimensional tungsten oxide nanosheets with extraordinary sensitivity and selectivity to NO2. Nanoscale 2017, 9, 19162–19175. DOI:10.1039/c7nr05403c [Google Scholar]
-
Yang L, Heinlein J, Hua C, Gao R, Hu S, Pfefferle L, et al. Emerging dual-functional 2D transition metal oxides for carbon capture and utilization: A review. Fuel 2022, 324, 124706. DOI:10.1016/j.fuel.2022.124706 [Google Scholar]
-
Azmi NZM, Buthiyappan A, Patah MFA, Rashidi NA, Raman AAA. Enhancing the CO2 adsorption with dual functionalized coconut shell-hydrochar using chlorella microalgae and metal oxide: Synthesis, physicochemical properties & mechanism evaluations. J. Cleaner Prod. 2024, 463, 142736. DOI:10.1016/j.jclepro.2024.142736 [Google Scholar]
-
Ghaemi A, Mashhadimoslem H, Zohourian Izadpanah P. NiO and MgO/activated carbon as an efficient CO2 adsorbent: characterization, modeling, and optimization. Int. J. Environ. Sci. Technol. 2022, 19, 727–746. DOI:10.1007/s13762-021-03582-x [Google Scholar]
-
Nowrouzi M, Younesi H, Bahramifar N. Superior CO2 capture performance on biomass-derived carbon/metal oxides nanocomposites from Persian ironwood by H3PO4 activation. Fuel 2018, 223, 99–114. DOI:10.1016/j.fuel.2018.03.035 [Google Scholar]
-
Liu W-J, Jiang H, Tian K, Ding Y-W, Yu H-Q. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013, 47, 9397–9403. DOI:10.1021/es401286p [Google Scholar]
-
Gao N, Chen K, Quan C. Development of CaO-based adsorbents loaded on charcoal for CO2 capture at high temperature. Fuel 2020, 260, 116411. DOI:10.1016/j.fuel.2019.116411 [Google Scholar]
-
Li Q, Guo J, Xu D, Guo J, Ou X, Hu Y, et al. Electrospun N-doped porous carbon nanofibers incorporated with NiO nanoparticles as free-standing film electrodes for high-performance supercapacitors and CO2 capture. Small 2018, 14, 1704203. DOI:10.1002/smll.201704203 [Google Scholar]
-
Xu X, Xu Z, Gao B, Zhao L, Zheng Y, Huang J, et al. New insights into CO2 sorption on biochar/Fe oxyhydroxide composites: Kinetics, mechanisms, and in situ characterization. Chem. Eng. J. 2020, 384, 123289. DOI:10.1016/j.cej.2019.123289 [Google Scholar]
-
Hosseini S, Bayesti I, Marahel E, Babadi FE, Abdullah LC, Choong TS. Adsorption of carbon dioxide using activated carbon impregnated with Cu promoted by zinc. J. Taiwan Inst. Chem. Eng. 2015, 52, 109–117. DOI:10.1016/j.jtice.2015.02.015 [Google Scholar]
-
Lahuri AH, Rahim AA, Nordin N, Adnan R, Jaafar NF, Taufiq-Yap YH. Comparative studies on adsorption isotherm and kinetic for CO2 capture using iron oxide impregnated activated carbon. Catal. Today 2023, 418, 114111. DOI:10.1016/j.cattod.2023.114111 [Google Scholar]
-
Chen G, Wang F, Wang S, Ji C, Wang W, Dong J, et al. Facile fabrication of copper oxide modified activated carbon composite for efficient CO2 adsorption. Korean J. Chem. Eng. 2021, 38, 46–54. DOI:10.1007/s11814-020-0684-1 [Google Scholar]
-
Li M, Huang K, Schott JA, Wu Z, Dai S. Effect of metal oxides modification on CO2 adsorption performance over mesoporous carbon. Microporous Mesoporous Mater. 2017, 249, 34–41. DOI:10.1016/j.micromeso.2017.04.033 [Google Scholar]
-
Abdedayem A, Guiza M, Ouederni A. Copper supported on porous activated carbon obtained by wetness impregnation: effect of preparation conditions on the ozonation catalyst’s characteristics. Comptes Rendus Chim. 2015, 18, 100–109. DOI:10.1016/j.crci.2014.07.011 [Google Scholar]
-
Wang X, Cheng H, Ye G, Fan J, Yao F, Wang Y, et al. Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review. Chemosphere 2022, 287, 131995. DOI:10.1016/j.chemosphere.2021.131995 [Google Scholar]
-
Guo Y, Tan C, Wang P, Sun J, Li W, Zhao C, et al. Magnesium-based basic mixtures derived from earth-abundant natural minerals for CO2 capture in simulated flue gas. Fuel 2019, 243, 298–305. DOI:10.1016/j.fuel.2019.01.108 [Google Scholar]
-
Guo Y, Tan C, Sun J, Li W, Zhang J, Zhao C. Biomass ash stabilized MgO adsorbents for CO2 capture application. Fuel 2020, 259, 116298. DOI:10.1016/j.fuel.2019.116298 [Google Scholar]
-
Tan B, Cheng G, Zhu X, Yang X. Experimental study on the physisorption characteristics of O2 in coal powder are effected by coal nanopore structure. Sci. Rep. 2020, 10, 6946. DOI:10.1038/s41598-020-63988-4 [Google Scholar]
-
Arjona-Jaime P, Morales-Ospino R, Izquierdo MT, Chazaro-Ruiz LF, Celzard A, Rangel-Mendez R, et al. Ca-modified biochar by microwave irradiation for robust CO2 capture under realistic flue gas conditions. Chem. Eng. J. 2025, 525, 170349. DOI:10.1016/j.cej.2025.170349 [Google Scholar]
-
Goel C, Mohan S, Dinesha P. CO2 capture by adsorption on biomass-derived activated char: A review. Sci. Total Environ. 2021, 798, 149296. DOI:10.1016/j.scitotenv.2021.149296 [Google Scholar]
-
Li H, Zick ME, Trisukhon T, Signorile M, Liu X, Eastmond H, et al. Capturing carbon dioxide from air with charged-sorbents. Nature 2024, 630, 654–659. DOI:10.1038/s41586-024-07449-2 [Google Scholar]
-
Hsu K-J, Li S, Micari M, Chi H-Y, Villalobos LF, Huang S, et al. Graphene membranes with pyridinic nitrogen at pore edges for high-performance CO2 capture. Nat. Energy 2024, 9, 964–974. DOI:10.1038/s41560-024-01556-0 [Google Scholar]
-
Eichler JE, Leonard H, Yang EK, Smith LA, Lauro SN, Burrow JN, et al. Dual-cation activation of N-enriched porous carbons improves control of CO2 and N2 adsorption thermodynamics for selective CO2 capture. Adv. Funct. Mater. 2024, 34, 2410171. DOI:10.1002/adfm.202410171 [Google Scholar]