Construction and Applications of Efficient, Oxygen-Tolerant Triplet-Triplet Annihilation Upconversion Materials
Received: 12 November 2025 Revised: 01 December 2025 Accepted: 11 December 2025 Published: 18 December 2025
© 2025 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Upconversion luminescence, a typical anti-Stokes shift optical phenomenon that converts low-energy photons into high-energy photons through multi-photon absorption mechanisms, has served as a revolutionary tool for modern biomedical research [1,2,3]. Compared to traditional fluorescent materials, these materials demonstrate unique advantages in cutting-edge fields, including spatiotemporally resolved imaging at the single-cell level [4,5], precise modulation of neural circuits [6], and tracking of tumor immune microenvironments [7]. Although current mainstream lanthanide-doped inorganic upconversion nanoparticles can achieve conversion from 980/808 nm near-infrared light to ultraviolet-visible light, their intrinsic limitations, such as low quantum efficiency (typically <1%) [8,9] and potential risks of rare-earth ion bioaccumulation [10], severely constrain their expanded applications in biomedicine [11]. The groundbreaking development of TTA-UC systems based on organic molecules offers an innovative strategy to overcome these challenges [12,13,14]. With their exceptionally high upconversion quantum yields, low excitation power thresholds at the milliwatt level, and programmable spectral tuning characteristics, these systems have inaugurated a new era for upconversion technology [13,14,15]. The TTA-UC system consists of a photosensitizer and two annihilators. The process begins with the photosensitizer absorbing low-energy photons and transferring to its singlet excited state, followed by intersystem crossing to the triplet excited state. Through sensitization by the triplet-state photosensitizer, the annihilator is promoted to its triplet state. Two triplet annihilator molecules then undergo triplet-triplet annihilation (TTA), whereby one molecule returns to the ground state while the other transitions to the singlet excited state, subsequently emitting a high-energy photon [14,15]. In fundamental photochemistry, the development of novel photosensitizers is crucial for advancing TTA-UC, and several molecular design strategies have been proposed to accelerate intersystem crossing rates [16,17,18,19,20]. Moreover, through sophisticated structural optimization of both photosensitizers and annihilators, multiple high-performance TTA-UC systems have been fabricated [21,22,23,24,25]. Particularly for TTA-UC in the visible region, upconversion efficiency can exceed 30% [26,27]. In practical applications, TTA-UC systems coupled with light-absorbing photocatalysts can extend the excitation wavelength of photocatalysis into the near-infrared region, thereby avoiding side reactions induced by substrates or intermediates. This approach provides new opportunities for large-volume photoredox catalysis [22,28,29,30]. In solar energy harvesting, near-infrared TTA-UC directly converts low-energy photons into high-energy photons, improving the power conversion efficiency of photovoltaic devices [31,32,33,34,35,36]. For biomedicine, TTA-UC enables background-free bioimaging with significantly improved signal-to-noise ratios [37,38,39,40]. Furthermore, when integrated with photoactive molecules or optogenetic tools, TTA-UC facilitates near-infrared light-driven targeted therapy and regulation of cellular signaling pathways [41,42,43,44,45,46,47]. In biosensing, TTA-UC has demonstrated new background-free sensing technologies for the detection of key biomolecules, including oxygen, glucose, sucrose hydrolase, and sarcosine [48,49,50,51,52,53].
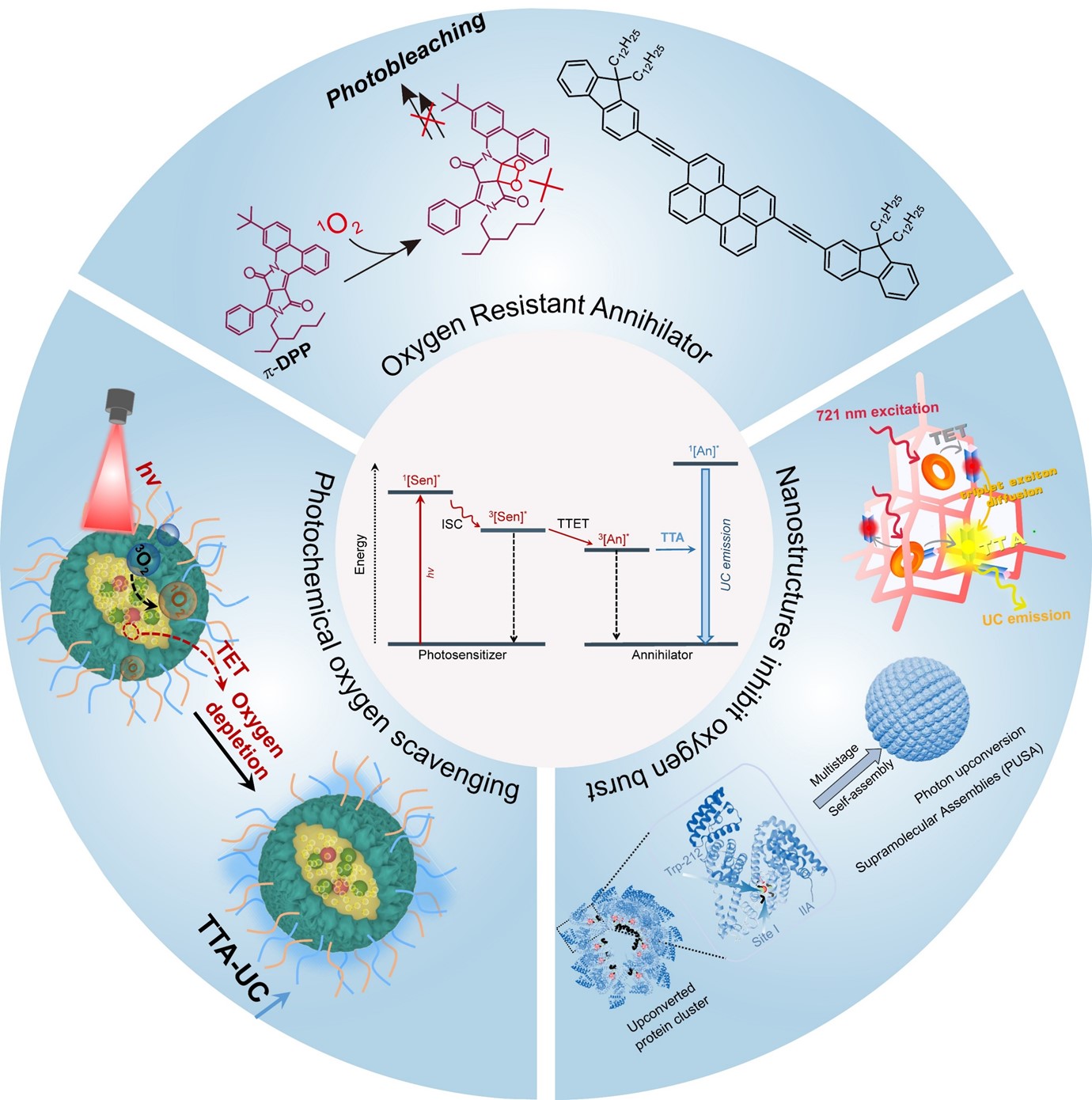
However, molecular oxygen, which acts as a natural triplet quencher, not only accelerates the dissipation of triplet excitons through electronic energy transfer but can also be photosensitized to form highly reactive singlet oxygen (1O2), thereby inducing oxidative degradation of the photosensitizer/annihilator [54,55,56,57]. Nevertheless, the practical application of TTA-UC requires effective strategies to circumvent oxygen-induced quenching of the upconversion luminescence. To address this challenge, the construction of oxygen-resistant TTA-UC systems necessitates synergistic innovations across two dimensions: molecular design and microenvironment modulation. At the molecular level, enhancing the intrinsic photostability of TTA-UC molecules through rational structural design of photosensitizers and annihilators can suppress luminescence quenching caused by photobleaching. At the material level, introducing oxygen scavengers to create a hypoxic microenvironment or constructing specific nanostructures to promote intermolecular triplet energy transfer rate can effectively prevent oxygen-induced quenching of molecular triplet states. Particularly noteworthy is that in nanoconfined environments, the inevitable molecular stacking between photosensitizers and annihilators may lead to quenching of excited states. Therefore, for developing highly efficient TTA-UC nanoparticles, establishing a hypoxic microenvironment within the nanoparticles is expected to inhibit quenching caused by intermolecular stacking. This review will elaborate on progress in oxygen-resistant and efficient TTA-UC materials from three aspects.
2. Enhanced Photostability of TTA-UC Molecules Promotes Oxygen-Resistant Material Development
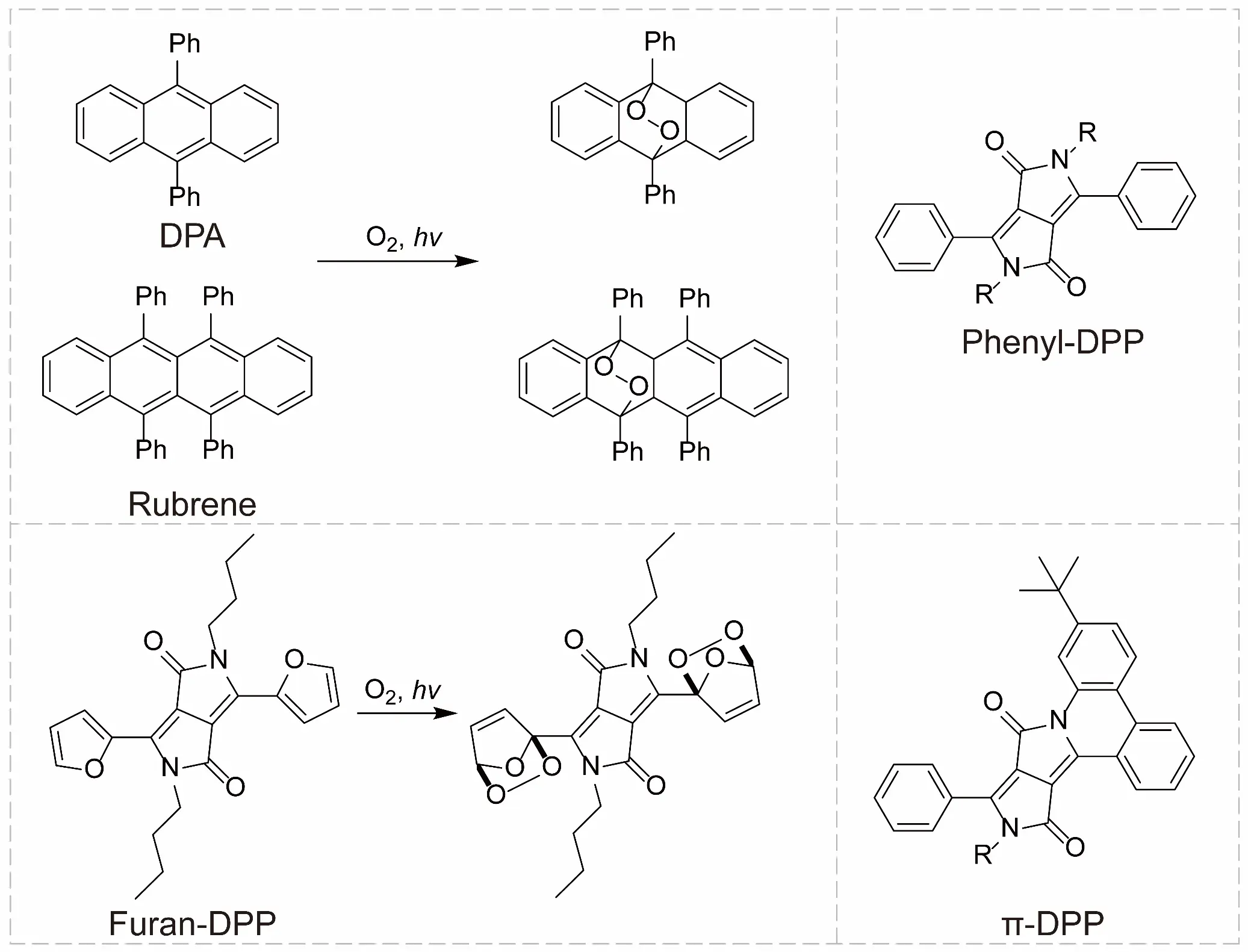
The improvement of photostability in TTA-UC molecular systems represents a crucial technical approach for constructing oxygen-resistant TTA-UC materials. In TTA-UC, triplet energy transfer between photosensitizers or annihilators and molecular oxygen can lead to the generation of singlet oxygen (1O2). This highly reactive oxygen species disrupts molecular structures via oxidative degradation pathways, significantly compromising the photostability of TTA-UC [58,59]. Taking classical annihilators such as 9,10-diphenylanthracene and rubrene as examples, their polycyclic aromatic hydrocarbon frameworks are prone to [2+2] cycloaddition reactions with 1O2, resulting in irreversible molecular structural damage (Figure 1) [58]. To address this limitation, introducing electron-withdrawing groups (e.g., cyano groups) into the annihilator’s molecular structure can substantially elevate its oxidation potential, thereby suppressing its reactivity toward 1O2. Experimental studies demonstrate that cyano-functionalized tetracene derivatives enhance their photostability by more than fourfold compared to the original system, while simultaneously enabling precise tuning of excited-state energy levels [58]. This strategy has also proven effective in significantly improving the photostability of perylene derivatives [60].
In recent years, diketopyrrolopyrrole (DPP)-based annihilators have attracted significant attention due to their exceptional spectral tunability. However, although heterocycle-substituted DPP derivatives (e.g., thiophene/furan-substituted) enable near-infrared-to-visible TTA-UC, their photostability remains limited by the intrinsic instability of the heterocyclic units (Figure 1) [61]. Systematic studies of energy levels revealed that phenyl-substituted DPP possess T1 energy levels between 1.3 and 1.4 eV, contrary to the previously reported value of 1.1 eV [52,61], a finding that challenges conventional understanding. When paired with the NIR photosensitizer PtTNP (T1 = 1.35 eV), phenyl-DPP not only satisfies the energy level matching requirement for annihilators but also achieves a high upconversion quantum yield of 9.4% [58]. To further modulate the excited-state energy levels of phenyl-DPP derivatives, new annihilator structures were synthesized via palladium-catalyzed annulation reactions. These molecules not only retain the excellent photostability of phenyl-DPP but also exhibit reduced singlet and triplet energy levels due to the extended π-conjugated system. Furthermore, the exceptional photostability of this annihilator was demonstrated in both air-saturated toluene solutions and polystyrene films. Compared to conventional rubrene and heterocycle-substituted DPP annihilators, the new annihilator exhibits superior photostability [58].
Traditional perylene-based compounds exhibit excellent luminescence efficiency and photostability due to their highly conjugated structures, as well as tunable optical properties, thermal stability, and ease of functionalization. However, the development of efficient near-infrared (NIR) light-activated TTA-UC has been limited by the fact that the triplet energy level (T1 = 1.53 eV) of Py-based annihilators is significantly higher than that of sensitizers such as PdTNP, resulting in a triplet–triplet energy transfer (TTET) process that is an unfavorable endothermic reaction, thereby hindering efficient upconversion luminescence. Through Sonogashira cross-coupling reactions, aryl–alkynyl units were introduced onto the perylene core, leading to the synthesis of a new series of annihilators (Py1, Py2, Py3, Py4, and Py5) (Figure 2). This strategy effectively extends the π-conjugated system of the molecules and reduces their triplet energy levels, enabling them to meet the energy level matching requirement with the photosensitizer PdTNP. When combined with PdTNP (10 μM), the diaryl–alkynyl-substituted perylene annihilators achieved a high upconversion quantum yield of 14.1% [22]. These perylene derivatives not only enable precise modulation of singlet and triplet energy levels but also successfully transform the TTA-UC system from an endothermic process into a more favorable exothermic one, while maintaining excellent photostability.
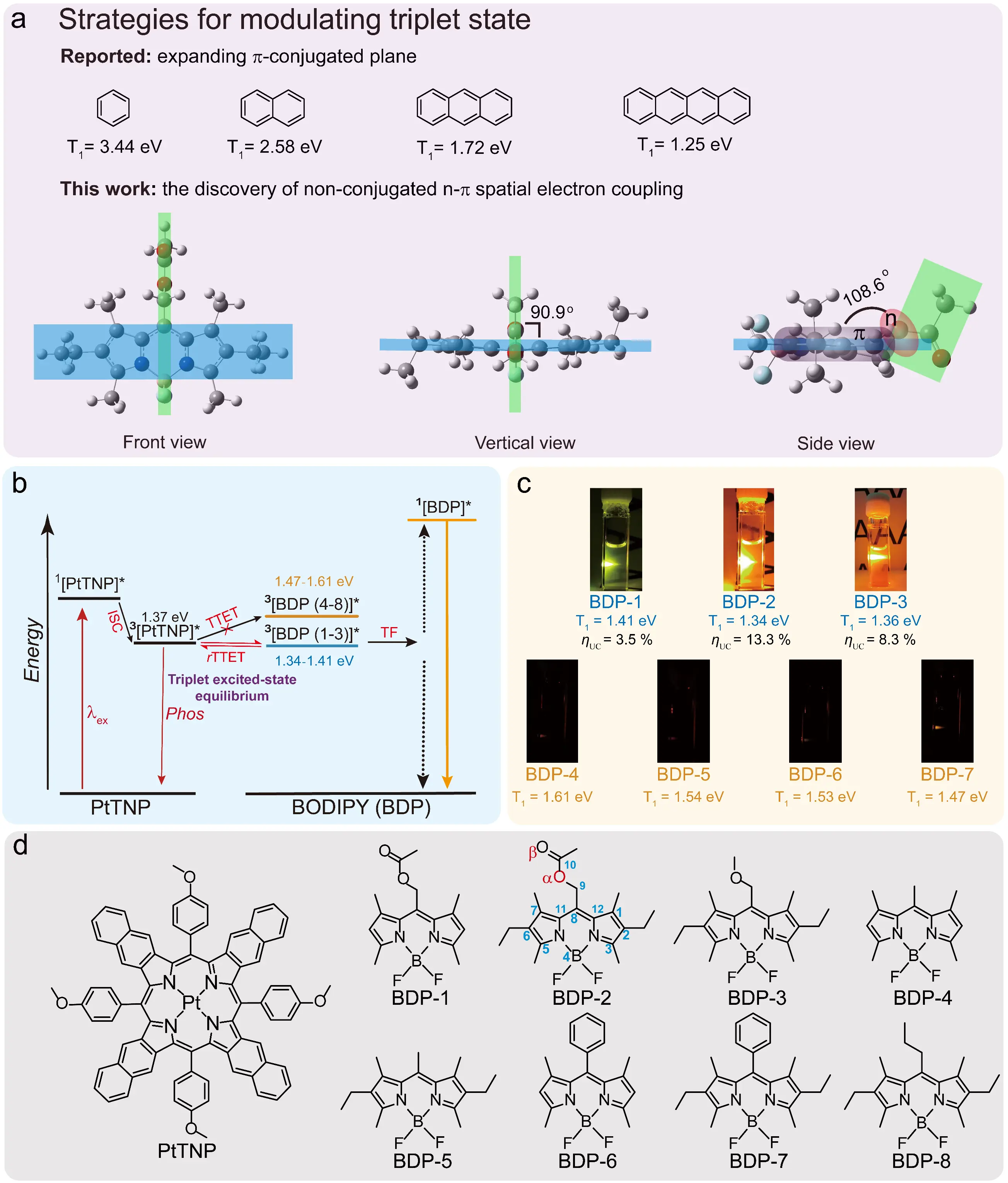
In contrast to polycyclic aromatic annihilators, boron dipyrromethene (BODIPY) derivatives exhibit superior photostability. However, their intrinsically high triplet energy levels (>1.60 eV) hinder efficient energy matching with NIR-absorbing photosensitizers, making NIR-to-visible TTA-UC challenging [62]. Through the strategic modulation facilitated by the stereoelectronic effect of an 8-ethoxycarbonyl group, synergistic interaction between its lone pair electrons and the π-system of the BODIPY core results in a reduction of both the singlet and triplet energy levels to 2.22 eV and 1.34 eV, respectively (Figure 3a–c). When coupled with the NIR-absorbing photosensitizer PtTNP, this system achieves a high upconversion quantum yield of 13.3%. Further polymer-encapsulated PtTNP/BDP-2 complexes were successfully fabricated into oxygen-resistant TTA-UC films, within which intense upconversion luminescence is clearly observed. This finding breaks the inherent limitation of conventional BODIPY molecules with elevated triplet energy levels (>1.60 eV), establishing a new strategy for the design of NIR-driven systems (Figure 3d) [63].
The aforementioned research findings demonstrate that the synergistic combination of electron-withdrawing group modification and molecular conformational engineering can simultaneously achieve intrinsic enhancement of annihilator photostability and precise regulation of excited-state energy levels. This approach provides an innovative solution for constructing oxygen-resistant TTA-UC systems and establishes a critical foundation for developing oxygen-resistant TTA-UC materials.
3. Fabrication of Oxygen-Resistant TTA-UC Nanoparticles via Reductive Oil-Droplet Strategy
The luminescence quenching effect of dissolved oxygen on TTA-UC nanoparticles in aqueous solutions represents a critical challenge for practical applications. Generally, external means cannot be employed to remove dissolved oxygen molecules from the aqueous environment in real-world scenarios; instead, oxygen molecules within or around the nanoparticles must be eliminated via specific chemical reactions to restore upconversion luminescence. Consequently, utilizing reductive oil droplets as oxygen scavengers has emerged as a primary technical pathway for fabricating oxygen-resistant TTA-UC nanoparticles. Currently, these reductive oil droplets typically comprise molecules that readily react with singlet oxygen. Under light excitation, the sensitizer photosensitizes dissolved oxygen to generate singlet oxygen, which subsequently reacts with the reductive oil droplets to create a localized hypoxic microenvironment, further suppressing oxygen-induced triplet-state quenching. In early studies, dextran-modified bovine serum albumin (BSA) was used as a coating agent to encapsulate soybean oil containing unsaturated double bonds, thereby constructing a reductive microenvironment (Figure 4a). Upon red light irradiation, the singlet oxygen generated by photosensitizers (e.g., PtTPBP) reacted with the double bonds, establishing local hypoxia within nanoparticles and enabling the observation of upconversion luminescence in oxygen-saturated aqueous solutions. Although this pioneering work first validated the feasibility of constructing hypoxic microenvironments, the low density of soybean oil and suboptimal nanoparticle size (>100 nm) significantly compromised both water dispersibility and biocompatibility, failing to meet practical application requirements. This groundbreaking work laid the foundation for the development of oxygen-resistant TTA-UC nanoparticles [64].
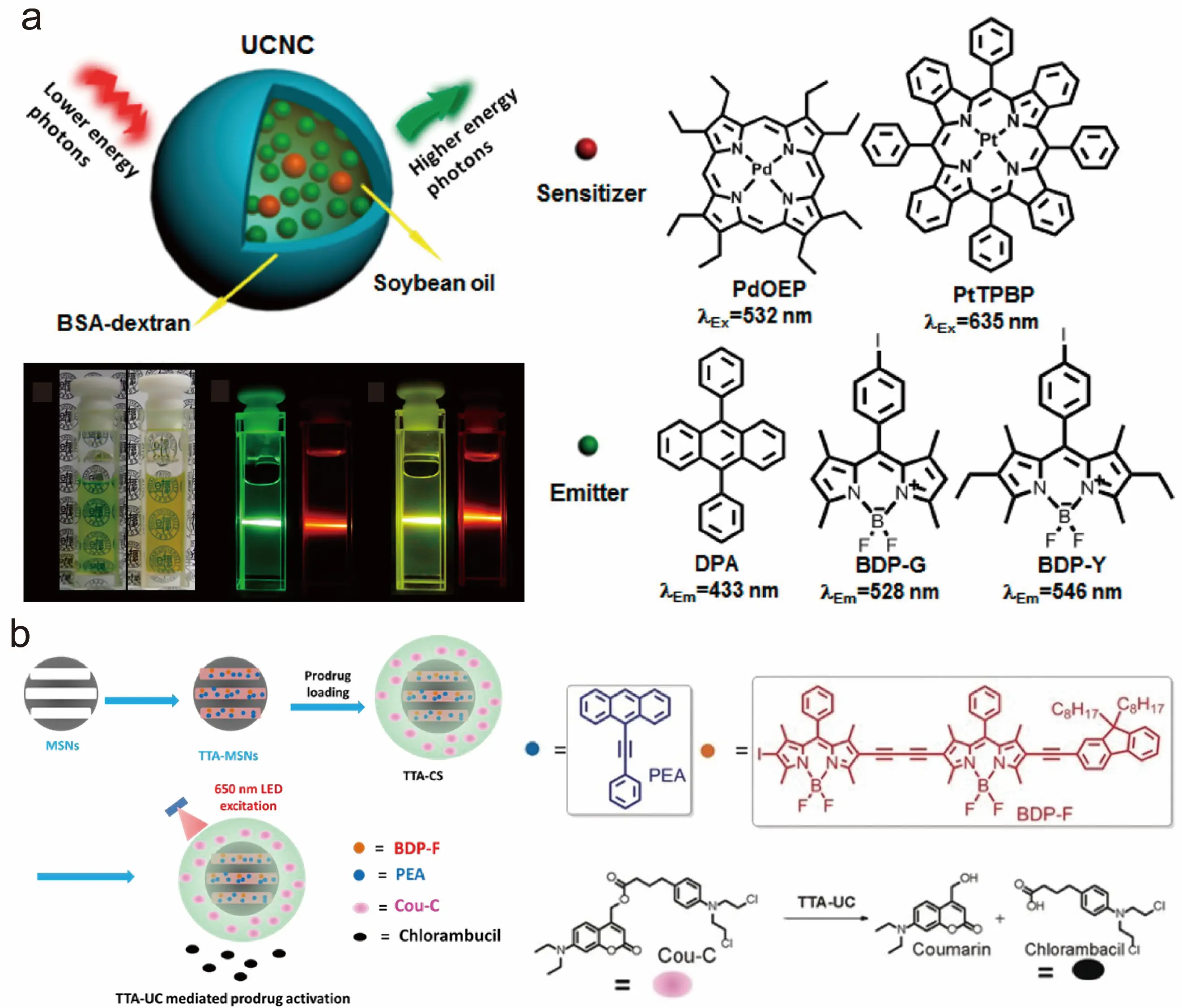
Figure 4. Early TTA-UC nanoparticle encapsulation strategy [47,64]. (a) BSA-capped TTA-UC nanoparticles [64]; (b) TTA-UC nanoparticles encapsulated by the mesoporous silica template method [47]. Panels (a) adapted from ref. [64]. Copyright 2013 American Chemical Society. Panels (b) adapted from ref. [47]. Copyright 2017 Wiley–VCH.
To address these limitations, we developed a mesoporous silica templating method to encapsulate TTA-UC molecules loaded with methyl oleate within ordered mesopores, yielding monodisperse oxygen-resistant TTA-UC nanoparticles with uniform particle size (218 nm ± 16 nm). Innovatively, unsaturated alkenes were covalently conjugated to amphiphilic polymer chains, facilitating the formation of nanoparticles approximately 30 nm in size via self-assembly. Furthermore, annihilators were used as photoprotecting groups to conjugate to drug molecules and synthesize prodrugs. Through the TTA mechanism, the wavelength for photocleavable prodrug release was significantly extended into the near-infrared region (Figure 4b). However, constrained by the insufficient alkene content in the polymer matrix, the system requires prolonged irradiation to activate upconversion luminescence, thereby limiting its practical application [47].
The use of solvents with oxygen-scavenging capabilities represents a common strategy to achieve oxygen-tolerant TTA-UC. For instance, in a DMSO solution containing PtOEP/DPA, singlet oxygen generated through photosensitization oxidizes trace amounts of DMSO, enabling in situ deoxygenation and resulting in efficient oxygen-tolerant TTA-UC. Besides DMSO, liquid sulfoxide solvents (e.g., TMSO) and liquid cyclic urea solvents (e.g., DMPU, DMI) can also achieve similar deoxygenation effects through analogous mechanisms [65]. Similarly, for phosphorescent gold(I) complexes, DMSO can trap photosensitized singlet oxygen, leading to enhanced phosphorescence emission [66]. Furthermore, the anti-Stokes shift of Os-phen/DPA in deoxygenated CH2Cl2 can reach 1.14 eV; to enable operation under ambient conditions, distinct upconverted emission remains observable in a mixed DMSO/CH2Cl2 solvent system [67].
To accelerate the oxygen scavenging rate, we systematically evaluated the oxygen scavenging efficiencies of various organic amines (aryl amines, alkyl primary/secondary/tertiary amines) through photo-oxidation reactions (Figure 5a). Using PdTPBP as the photosensitizer and C12-alkyl modified perylene as the annihilator (Figure 5b), the recovery kinetics of upconversion luminescence were investigated by monitoring intensity changes in DMF solutions containing PdTPBP/perylene and organic amines under red light irradiation. Experimental results indicated that no upconversion luminescence was observed in TTA-UC solutions containing aryl primary amines under illumination, demonstrating their inability to function as oxygen scavengers. In solutions containing alkyl primary and secondary amines, oxygen scavenging is achieved via a photosensitized singlet-oxygen trapping mechanism, with the upconversion luminescence recovery rate in these systems being less than 200 s. Although upconversion luminescence was generated in solutions containing alkyl tertiary amines, the recovery time was significantly longer, and the intensity was lower compared to alkyl primary and secondary amines. These findings suggest distinct mechanisms for upconversion generation among the three alkyl amine types. Mechanistic studies on photo-induced electron transfer and triplet energy transfer mechanisms of PdTPBP (Figure 5c) revealed that alkyl primary and secondary amines primarily facilitate oxygen depletion by photosensitized singlet oxygen generation, thereby eliminating dissolved oxygen. In contrast, solutions containing alkyl tertiary amines involved photo-induced electron transfer between the sensitizer and the amine, leading to upconversion quenching (Figure 5d). Furthermore, amine-rich polyethyleneimine was conjugated to polymer surfaces to synthesize amphiphilic polymers. Using butyl benzoate as the oil phase to suppress PdTPBP/perylene aggregation, oxygen-resistant TTA-UC nanoparticles with upconversion efficiency as high as 1.0% were fabricated, exhibiting a small particle size of only 17.2 nm ± 1.5 nm. The universality of this method for preparing oxygen-resistant TTA-UC nanoparticles was validated by encapsulating other TTA-UC molecules with different excitation and emission wavelengths, preparing nanoparticles with tunable excitation/emission profiles (Figure 5e) [68].
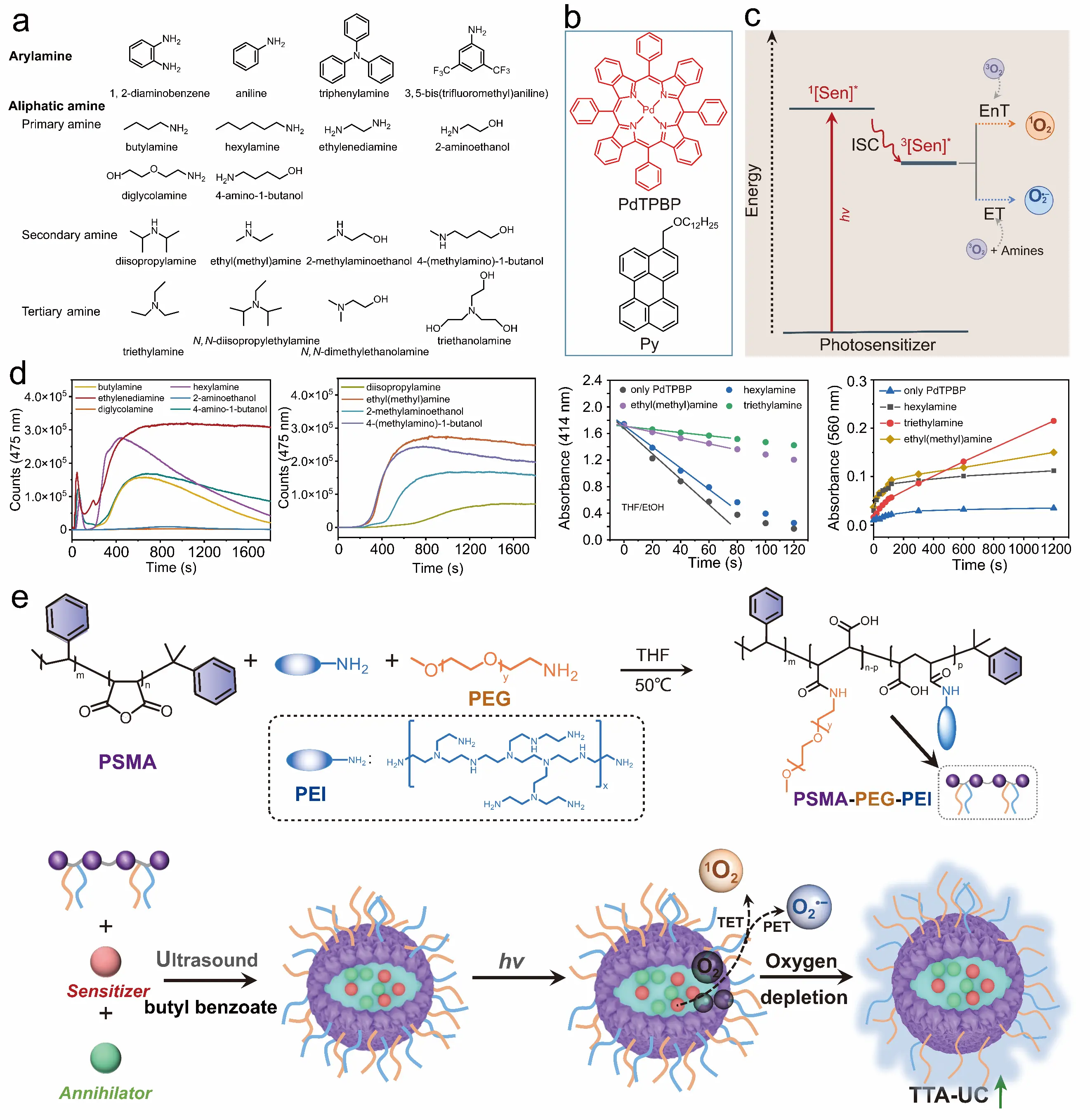
Figure 5. Enhancement of TTA-UC luminescence by photoredox reactions of organic amines [68]. (a) Structures of 18 organic amines; (b) Structures of the photosensitizer PdTPBP and the annihilator perylene; (c) The dual role of organic amines in photo-oxidation inhibits the oxygen-induced triplet quenching; (d) TTA-UC kinetics and generation of single oxygen (414 nm) and superoxide anion (560 nm) involved in organic amines; (e) Polyethyleneimine-coated TTA-UC nanoparticles. Reproduced with permission from ref. [68]. Copyright 2024 American Chemical Society.
Although photo-oxidation of organic amines is an effective method for preparing oxygen-resistant TTA-UC nanoparticles via oxygen scavenging, these amines can induce hemolysis in blood cells and cause significant biotoxicity. Consequently, developing fabrication methods for oxygen-resistant TTA-UC nanoparticles with good biocompatibility is highly desirable. Systematic screening of photo-oxidation reactions across 20 natural amino acids (Figure 6a) revealed that methionine and histidine could rapidly eliminate dissolved oxygen, enabling prompt recovery of upconversion luminescence. Mechanistic studies indicated that singlet oxygen generated via photosensitization reacts with methionine and histidine under light irradiation, thereby reducing dissolved oxygen concentrations in solution (Figure 6b). To address this limitation, the inherent hydrophilicity of amino acids hinders their direct application as hydrophobic oil droplets. Therefore, hydrophobic methionine derivatives are synthesized via butyl-group conjugation via an amidation reaction, which are subsequently employed as a hydrophobic oil phase to dissolve the photosensitizer (PdTPBP) and annihilator (perylene) (Figure 6c). Using an amphiphilic polymer to encapsulate oil droplets containing PdTPBP/perylene, oxygen-resistant TTA-UC nanoparticles were successfully fabricated (Figure 6d) with a high upconversion quantum yield reaching 7.2%. More significantly, compared to using oleic acid as the reductive oil droplet, the methionine-based derivative enabled a much faster recovery of TTA-UC luminescence, exhibiting a 400-fold increase in the TTA-UC recovery rate [69].
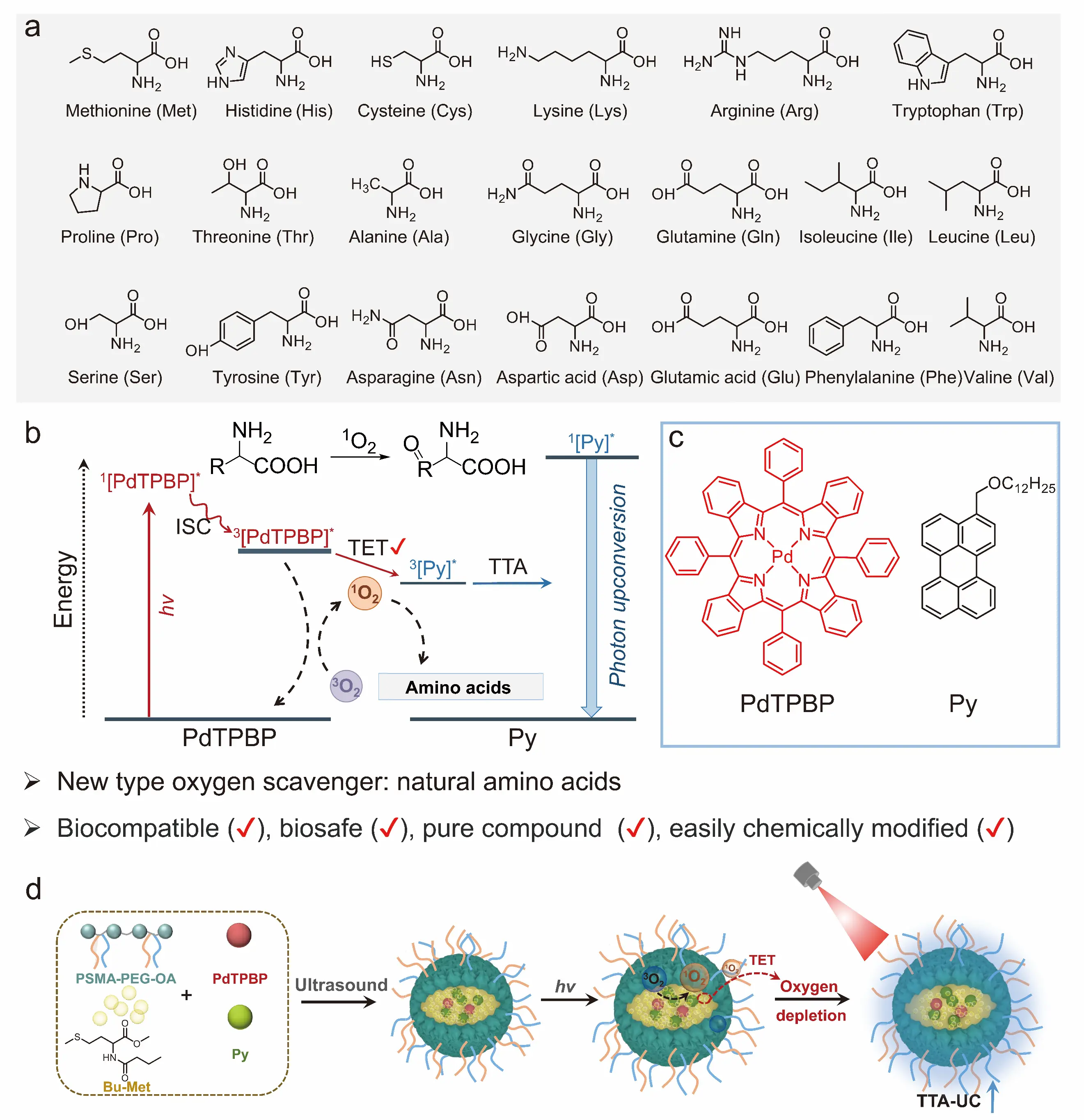
Figure 6. Enhancement of TTA-UC luminescence by photoredox reactions of amino acids [69]. (a) Structure of 20 natural amino acids; (b) Amino acid photo-oxidation recovery TTA-UC; (c) Structure of photosensitizer (PdTPBP) and annihilator (Py); (d) Preparation of oxygen-resistant TTA-UC nanoparticles with the addition of methionine derivatives. Panels (a–d) adapted from ref. [69]. Copyright 2025 Wiley–VCH.
4. Engineering Oxygen-Resistant TTA-UC Materials via Microstructure-Mediated Control of Intermolecular Triplet Energy Transfer Rates
TTA-UC fundamentally relies on intermolecular triplet energy transfer. However, molecular oxygen, acting as an efficient triplet quencher, significantly suppresses the luminescence intensity. Since TTA-UC depends on intermolecular Dexter energy transfer, which exhibits a strong distance dependence, the strategic design of specific microstructures can enhance the intermolecular triplet energy transfer rate to surpass that of triplet-state quenching by oxygen molecules. This strategy offers a feasible pathway to the construction of oxygen-resistant TTA-UC materials.
Metal-organic frameworks (MOFs), with their crystalline structures and dense molecular packing, are highly conducive to enhancing the intermolecular triplet energy transfer rate. For instance, the integration of palladium-substituted porphyrin photosensitizers and a DPA annihilator within a nanoscale MOF (Figure 7a) significantly boosts the intermolecular triplet energy transfer rate, achieving a triplet exciton diffusion constant as high as 7.7 × 10−6 cm2·s−1 and a triplet exciton diffusion length of up to 1.6 μm. In the nano-MOF, upconversion luminescence was observed even in air-saturated solutions, with an upconversion quantum yield of 1.28% [70].
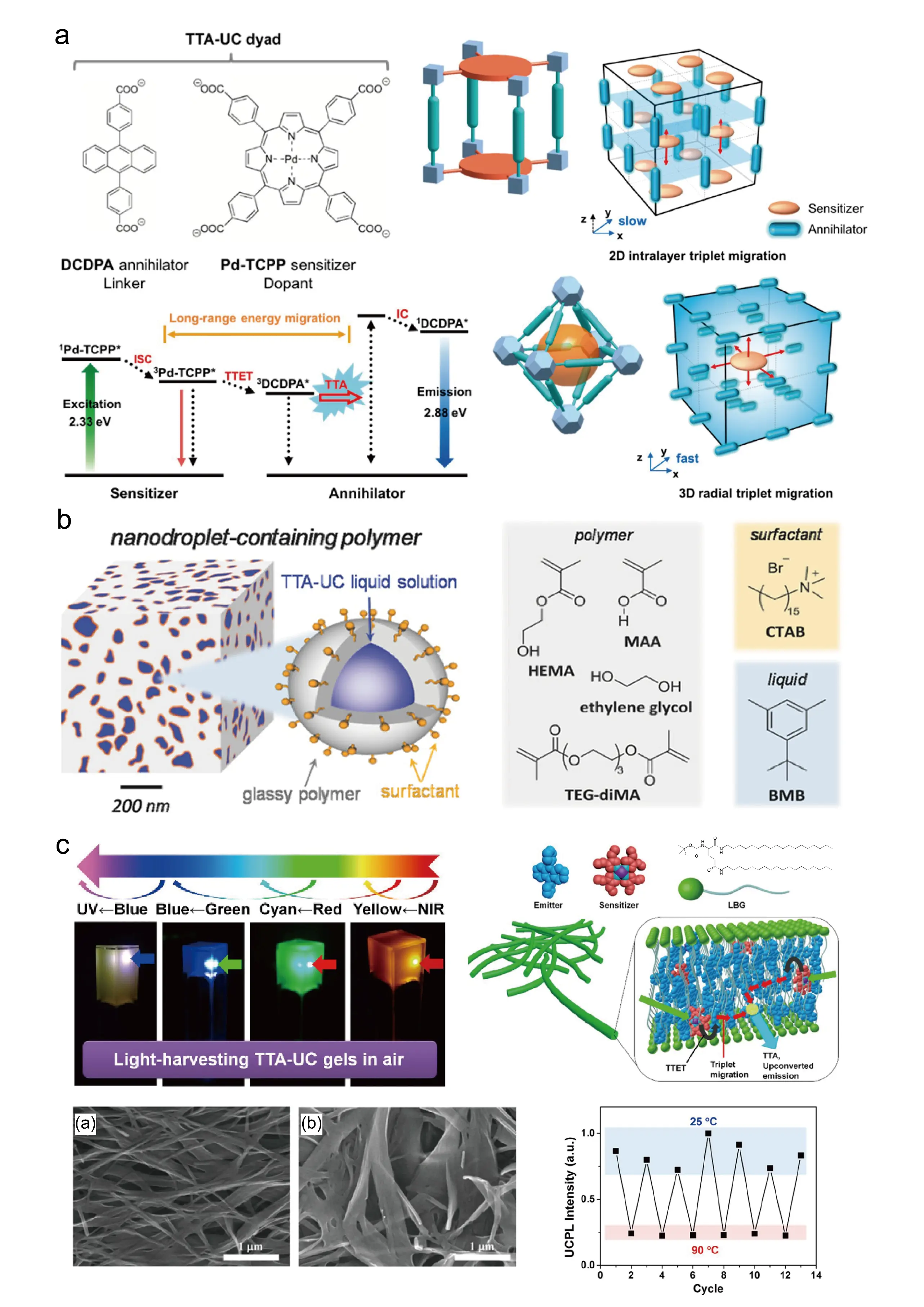
Figure 7. Photon upconversion in MOFs, glassy polymer, and supramolecular gel matrixes [70,71,72]. (a) Structural analysis and the triplet energy migration pathways in different types of TTA-UC MOF [70]; (b) Nanodroplet-containing polymers and chemical structures of the constituents forming the glassy polymer, the surfactant, and the liquid phase [71]; (c) Photon upconversion in supramolecular gel matrixes [72]. Panels (a) adapted from ref. [70]. Copyright 2018 American Chemical Society. Panels (b) adapted with permission from ref. [71]. Copyright 2017 Wiley–VCH. Panels (c) adapted from ref. [72]. Copyright 2015 American Chemical Society.
TTA-UC exhibits high upconversion efficiency in low viscosity solutions, but its efficiency significantly decreases in the more widely applicable solid materials. Through redox-initiated free-radical polymerization under ambient conditions, cross-linked glassy polymer materials containing nanodroplets were prepared in one step. This polymerization system consists of the hydrophilic monomers 2-hydroxyethyl methacrylate (HEMA) and methacrylic acid (MAA), the cross-linker triethylene glycol dimethacrylate (TEG-diMA), with the incorporation of a small amount of the lipophilic and low-volatility liquid 1-tert-butyl-3,5-dimethyl benzene (BMB), the surfactant cetyltrimethylammonium bromide (CTAB), along with ethylene glycol added as both refractive index modifier and polymer plasticizer, and the photosensitizer/annihilator pair PdOEP/DPA (Figure 7b). By confining PdOEP/DPA within the liquid nano-domains of the polymer, the intermolecular triplet energy transfer rate was significantly enhanced. This system demonstrates excellent oxygen tolerance, maintaining stable upconversion luminescence even under ambient air, with a constant upconversion efficiency of approximately 15%. This strategy indicates that encapsulating liquid TTA-UC systems within glassy polymers is an effective approach for constructing efficient and oxygen-resistant TTA-UC materials [71].
Polymers and natural biomolecules contain abundant functional groups such as carboxyl, amino, and hydroxyl groups, which readily form dense hydrogen-bonding networks. These networks significantly impede oxygen diffusion within these materials, thereby suppressing oxygen-induced triplet quenching and maintaining TTA-UC activity under ambient air. For example, the TTA-UC hydrogel system was fabricated using N,N′-bis(octadecyl)-l-boc-glutamic diamide (LBG) as the matrix, incorporating platinum octaethylporphyrin (PtOEP) and DPA. This hydrogel exhibited bright upconversion luminescence even in air (Figure 7c), achieving an upconversion efficiency of up to 3.5% [72]. The three-dimensional hydrogen-bonding network demonstrated distinct thermosensitive characteristics, with elevated temperatures inducing hydrogel network dissociation and a significant decrease in upconversion luminescence. This phenomenon unequivocally confirms the critical role of hydrogen-bond network formation in the development of oxygen-resistant TTA-UC materials [72,73,74]. Oxygen-tolerant TTA-UC materials can also be achieved by integration with hydrogels, as the abundant hydrogen-bonding networks within hydrogels suppress oxygen diffusion, thereby minimizing the quenching of TTA-UC by oxygen [75,76,77,78,79].
Solid-state TTA-UC materials are of significant importance for device processing and practical applications. The utilization of intermolecular van der Waals interactions enables the preparation of high-performance TTA-UC materials. For instance, during recrystallization, dissolving a trace amount of porphyrin sensitizer (approximately 0.001%) into a blue-fluorescent hydrocarbon annihilator molecular crystal yields uniformly colored and transparent solid-solution crystals, effectively suppressing sensitizer aggregation [80]. Similarly, doping a photosensitizer into a molten annihilator and then cooling can produce efficient, homogeneous solid-state upconversion materials [81]. Furthermore, based on surface temperature gradient control techniques, bicomponent organic polycrystalline films can be directly grown from mixed melts of sensitizers and annihilators [82]. Notably, co-dissolving multiple photosensitizers into the molecular crystal of the annihilator to broaden the absorption bandwidth can significantly enhance upconversion emission intensity, and the resulting crystals maintain excellent photostability under prolonged illumination for up to 50 h [83].
Nanoconfinement effects also serve as an effective strategy to enhance the intermolecular triplet energy transfer rate. Through multi-level hierarchical self-assembly of TTA-UC molecules with proteins, photon upconversion supramolecular assemblies were fabricated (Figure 8a–c). Within these protein-based nanoparticles, upconversion luminescence was observed even in the absence of reactive oxygen species scavenging units. Transient absorption studies revealed that this phenomenon originates from a significantly accelerated triplet energy transfer rate between the photosensitizer (PtTNP) and annihilator (DPP) in the protein-based supramolecular assembly, with the transfer rate surpassing that of oxygen-induced triplet quenching (Figure 8d). Most importantly, the oxygen-resistant TTA-UC nanoparticles constructed based on nanoconfinement effects exhibit a distinct upconversion generation mechanism compared to those relying on photo-oxidation reactions. Specifically, the maximum upconversion intensity is instantly observed upon irradiation and remains stable without temporal decay [52].
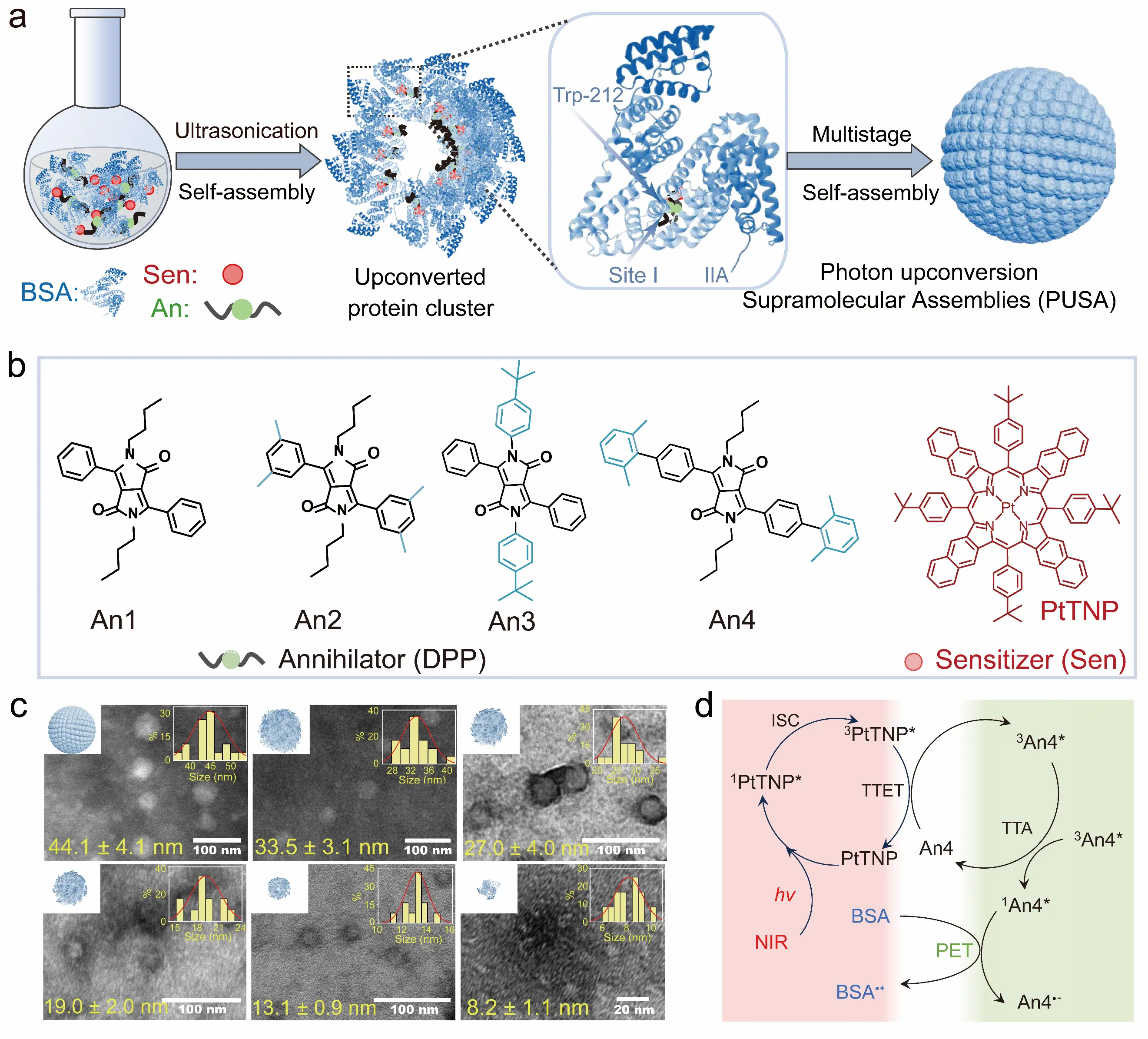
Figure 8. Natural protein photon upconversion supramolecular assemblies [52]. (a) Schematic process of protein self-assembly nanoparticle preparation; (b) Molecular structures of photosensitizer and annihilators; (c) Transmission electron microscopy (TEM) images of PtTNP/An4 assemblies containing different concentrations of BSA; (d) Schematic diagram of the photo-induced electron transfer from BSA to An4. Reproduced from ref. [52]. Copyright 2024 American Chemical Society.
Porous materials have become ideal platforms for fabricating efficient solid-state triplet-triplet annihilation upconversion (TTA-UC) materials due to their rigid frameworks, which facilitate long-range exciton migration. However, the triplet exciton diffusion constants in previously reported TTA-UC systems based on crystalline metal-organic frameworks (MOFs) still require improvement. This limitation stems from metal nodes in MOFs, which often act as exciton-quenching sites, resulting in shorter triplet exciton diffusion lengths and, consequently, suboptimal upconversion performance. Therefore, developing metal-free porous frameworks offers a significant advantage for enhancing exciton diffusion length.
In covalent organic frameworks (COFs), the energy levels of annihilator units can decrease as the π-conjugated plane of the framework extends, or framework vibrations may quench the annihilator’s excitons. To address this, we introduced steric hindrance into the annihilator units to disrupt the π-conjugation between the annihilators and the porous aromatic framework (PAF). This strategy establishes a more uniform triplet exciton energy landscape, thereby promoting exciton diffusion. For example, diketopyrrolopyrrole (DPP) was employed as the annihilator unit and integrated into the PAF-11 framework (Figure 9a). The photosensitizer palladium(II) tetraphenyltetrabenzoporphyrin (PdTBP) was subsequently introduced to prepare the organic upconversion material. Results demonstrate that DPP-PAF-2/PdTBP exhibits bright upconversion luminescence even under ambient conditions, with a high triplet exciton diffusion constant of 1.34 × 10−5 cm2·s−1 (Figure 9b). This enhancement is attributed to the incorporation of 3,5-dimethyl groups, which restrict free rotation between the DPP and phenyl units. This restriction leads to a more uniform triplet exciton energy landscape, enabling highly efficient exciton diffusion within the PAF framework. This work demonstrates that the rational design and utilization of PAFs can significantly enhance exciton diffusion rates, providing a new pathway for developing oxygen-resistant TTA-UC materials [53].
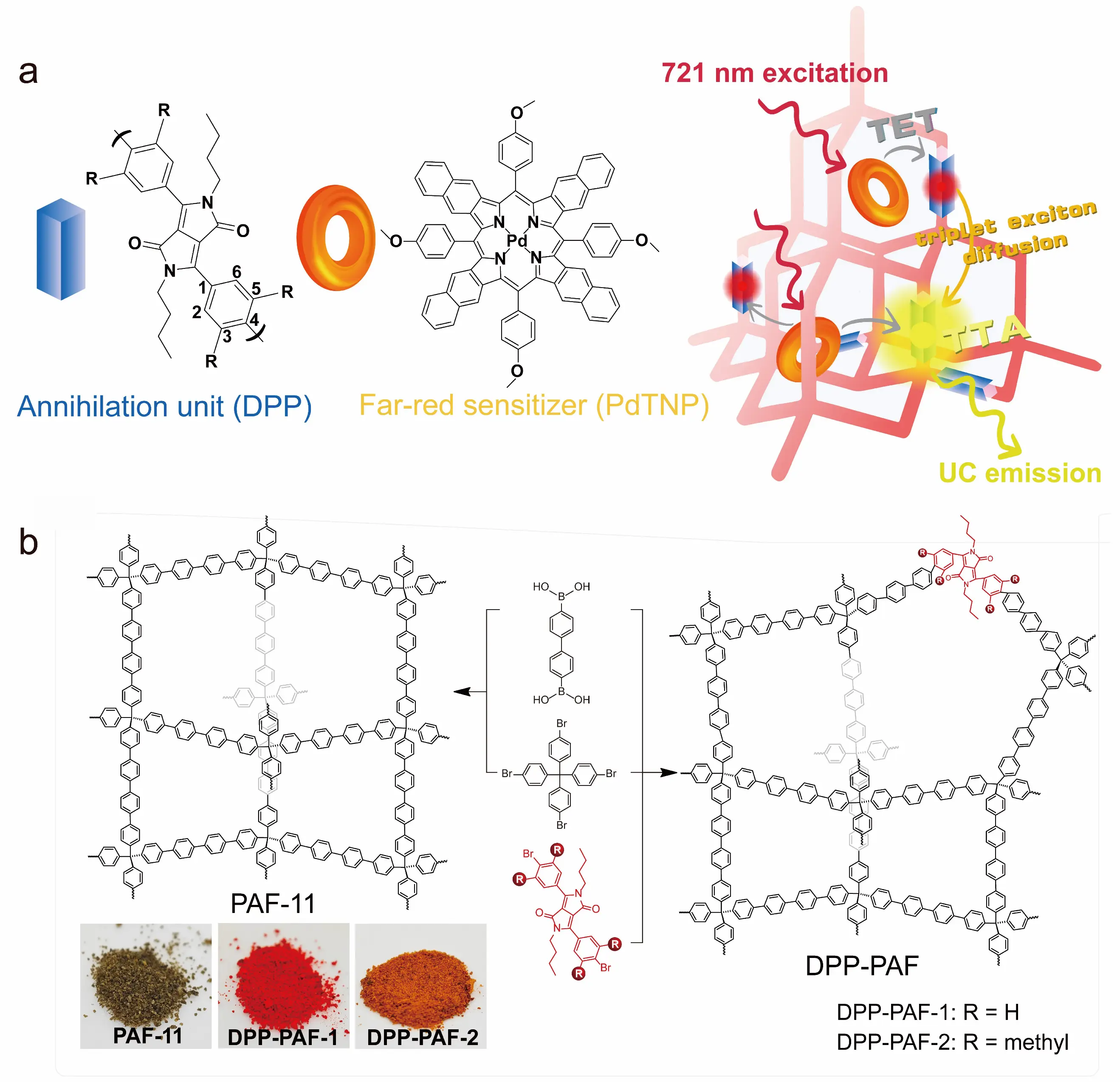
Figure 9. Schematic illustration of TTA-UC PAF and the synthetic procedure of PAF-11 and DPP-PAF [53]. (a) Schematic illustration of the 721 nm excitable triplet-triplet annihilation upconversion porous aromatic frameworks (TTA-UC PAFs); (b) Schematic illustration of the synthetic procedure of PAF-11 and DPP-PAF. The insets are images of PAF-11 and DPP-PAF. Panels (a,b) adapted with permission from ref. [53]. Copyright 2025 Wiley–VCH.
5. Challenges and Prospects
The current challenges in TTA-UC primarily encompass the following aspects:
- (1)
-
The upconversion quantum yields remain relatively low in the near-infrared region, which is fundamentally constrained by the energy gap law. This result dictates that sensitizers or annihilators with low-lying excited states predominantly undergo non-radiative decay, thereby suppressing intermolecular triplet energy transfer. Furthermore, the significantly expanded conjugated planes of NIR-absorbing sensitizers and annihilators make them highly susceptible to oxidative degradation by reactive oxygen species. These factors render NIR-light-activated TTA-UC systems particularly unstable.
- (2)
-
Regarding nanomaterial fabrication, although our group and others have developed a series of viable strategies for nanoparticle preparation, the existing methodologies still fail to meet the expanding application demands. For instance, current TTA-UC nanoparticles are inadequate for single-particle imaging applications. This is primarily because single-particle imaging requires extremely low nanoparticle concentrations, whereas most current TTA-UC nanoparticles are organic colloids. When their concentration falls below the critical micelle concentration, these nanoparticles tend to dissociate. Additionally, in vivo applications, the abundant biomacromolecules (e.g., proteins, nucleic acids, phospholipids) in biological systems can potentially undergo component exchange with the organic nanoparticle constituents, leading to structural dissociation. These factors represent key limitations for biomedical applications of TTA-UC.
- (3)
-
Surface functionalization of TTA-UC nanoparticles. To the best of our knowledge, no literature has specifically reported on the surface modification of TTA-UC nanoparticles. Although numerous surface modification strategies for nanoparticles have been documented, their applicability to TTA-UC nanoparticles remains to be validated. This is primarily because the current immaturity of TTA-UC nanoparticle technology, where the prepared nanoparticles still require further improvement. Effective surface functionalization of TTA-UC nanoparticles can significantly enhance their targeting specificity toward cells, tissues, and organs, which is crucial for advancing their biological applications.
To address the aforementioned challenges, we propose the following strategic approaches: Enhancing the performance of near-infrared (NIR) TTA-UC primarily focuses on mitigating the excited-state energy loss during the intersystem crossing (ISC) process of the sensitizers. Therefore, in-depth investigations of the ISC process of NIR photosensitizers and the development of novel photosensitizers possessing both NIR absorption and thermally activated delayed fluorescence characteristics are imperative. Since spin-state transitions in quantum dots (QDs) don’t entail significant excited-state energy loss, employing heavy-metal-free quantum dots, such as CuInSe2, as photosensitizers represents a viable strategy for advancing NIR TTA-UC [84]. Regarding the fabrication of TTA-UC nanomaterials, priority should be given to exploiting nanoconfinement effects to develop oxygen-resistant TTA-UC nanomaterials. This approach eliminates the dependency on reactive oxygen species scavengers, thereby simplifying nanoparticle composition and enhancing biocompatibility. Utilizing natural biomacromolecules for the preparation of TTA-UC nanomaterials can significantly reduce biotoxicity, facilitating broader biological applications. Concerning the surface modification of TTA-UC nanoparticles, the development of reliable modification methods is only meaningful once mature fabrication protocols are firmly established. Bioorthogonal reactions can be employed to conjugate targeting moieties, such as aptamers or antibodies, to the nanoparticle surface, thereby enhancing targeting specificity and achieving high signal-to-background ratio bioimaging in vivo. The modification of surface ligands on TTA-UC nanoparticles offers a strategy for constructing diverse bioprobes, enabling the background-free detection of malignant disease biomarkers.
As a new-generation organic upconversion system, TTA-UC has demonstrated unique application potential across diverse fields, including biomedicine, chemistry, and materials science. The advancement of synthetic biology offers new possibilities for integrating TTA-UC with biological systems, although current applications in this field remain at a nascent stage. A key consideration in synthetic biology lies in the strategic localization and functional assignment of TTA-UC. For instance, in applications aimed at photocatalytic CO2 to fuels using synthetic biology, the photosensitizer in TTA-UC systems may function as a unit for capturing low-energy photons to enhance photon utilization efficiency, while the annihilator could potentially serve as a photocatalyst or electron transfer mediator to improve CO2 photoconversion efficiency. In the field of bioimaging and diagnostics, a significant question is whether TTA-UC can emulate green fluorescent protein by enabling the design of genetically encoded upconversion proteins via synthetic biology. Currently, the genetically encoded small-molecule fluorophores can be achieved through unnatural amino acid insertion techniques. However, for TTA-UC, the simultaneous integration of both the photosensitizer and annihilator within a single protein significantly elevates the technical challenges in creating genetically encoded upconversion proteins. To mitigate the complexity of upconversion protein biosynthesis, employing photosensitizers and annihilators with simple molecular structures may represent the optimal strategy. Nevertheless, avoiding oxygen-induced quenching of TTA-UC and photo-induced charge transfer-mediated quenching between proteins and TTA-UC components will require careful protein structural optimization. Although no studies have yet reported the combined application of TTA-UC and synthetic biology, we believe that with the continuous refinement of TTA-UC molecules and material systems, the deep integration of synthetic biology with TTA-UC holds considerable promise.
Author Contributions
Conceptualization, Z.-W.L., H.-J.F. and L.H.; Writing—Original Draft Preparation, Z.-W.L. and F.Q.; Writing—Review & Editing, J.-Y.L., M.-Y.Z., Y.P., C.-X.P., W.-Y.L. and H.-J.F.; Supervision, H.-J.F. and L.H.; Funding Acquisition, H.-J.F. and L.H.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC) [22377063, 224B2405], Haihe Laboratory of Sustainable Chemical Transformations for financial support [24HHWCSS00020], and the Research Start-up Fund of Nankai University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
Richards BS, Hudry D, Busko D, Turshatov A, Howard IA. Photon Upconversion for Photovoltaics and Photocatalysis: A Critical Review. Chem. Rev. 2021, 121, 9165–9195. doi:10.1021/acs.chemrev.1c00034. [Google Scholar]
-
Deng Y, Jiang L, Huang L, Zhu T. Energy Flow in Hybrid Organic/Inorganic Systems for Triplet–Triplet Annihilation Upconversion. ACS Energy Lett. 2022, 7, 847–861. doi:10.1021/acsenergylett.1c02648. [Google Scholar]
-
Cheng X, Zhou J, Yue J, Wei Y, Gao C, Xie X, et al. Recent Development in Sensitizers for Lanthanide-Doped Upconversion Luminescence. Chem. Rev. 2022, 122, 15998–16050. doi:10.1021/acs.chemrev.1c00772. [Google Scholar]
-
Liu Q, Zhang Y, Peng CS, Yang T, Joubert L-M, Chu S. Single upconversion nanoparticle imaging at sub-10 W cm−2 irradiance. Nat. Photonics 2018, 12, 548–553. doi:10.1038/s41566-018-0217-1. [Google Scholar]
-
Zhang Y, Wen R, Hu J, Guan D, Qiu X, Zhang Y, et al. Enhancement of single upconversion nanoparticle imaging by topologically segregated core-shell structure with inward energy migration. Nat. Commun. 2022, 13, 5927. doi:10.1038/s41467-022-33660-8. [Google Scholar]
-
Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science 2018, 359, 679–684. doi:10.1126/science.aaq1144. [Google Scholar]
-
Wang F, Wen S, He H, Wang B, Zhou Z, Shimoni O, et al. Microscopic inspection and tracking of single upconversion nanoparticles in living cells. Light Sci. Appl. 2018, 7, 18007. doi:10.1038/lsa.2018.7. [Google Scholar]
-
Liu X, Yan CH, Capobianco JA. Photon upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1299–1301. doi:10.1039/c5cs90009c. [Google Scholar]
-
Wang X, Valiev RR, Ohulchanskyy TY, Ågren H, Yang C, Chen G. Dye-sensitized lanthanide-doped upconversion nanoparticles. Chem. Soc. Rev. 2017, 46, 4150–4167. doi:10.1039/c7cs00053g. [Google Scholar]
-
Gulzar A, Xu J, Yang P, He F, Xu L. Upconversion processes: versatile biological applications and biosafety. Nanoscale 2017, 9, 12248–12282. doi:10.1039/c7nr01836c. [Google Scholar]
-
Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. doi:10.1039/C4CS00177J. [Google Scholar]
-
Zhu X, Su Q, Feng W, Li F. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. doi:10.1039/c6cs00415f. [Google Scholar]
-
Chen X, Zhang X, Zhao Y. Metal-organic framework-based hybrids with photon upconversion. Chem. Soc. Rev. 2025, 54, 152–177. doi:10.1039/d4cs00571f. [Google Scholar]
-
Bharmoria P, Bildirir H, Moth-Poulsen K. Triplet-triplet annihilation based near infrared to visible molecular photon upconversion. Chem. Soc. Rev. 2020, 49, 6529–6554. doi:10.1039/d0cs00257g. [Google Scholar]
-
Xiao X, Tian W, Imran M, Cao H, Zhao J. Controlling the triplet states and their application in external stimuli-responsive triplet–triplet-annihilation photon upconversion: From the perspective of excited state photochemistry. Chem. Soc. Rev. 2021, 50, 9686–9714. doi:10.1039/D1CS00162K. [Google Scholar]
-
Zhao J, Xu K, Yang W, Wang Z, Zhong F. The triplet excited state of Bodipy: Formation, modulation and application. Chem. Soc. Rev. 2015, 44, 8904–8939. doi:10.1039/c5cs00364d. [Google Scholar]
-
Zhao J, Wu W, Sun J, Guo S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. doi:10.1039/c3cs35531d. [Google Scholar]
-
Leung C-F, Lau T-C. Organic photosensitizers for catalytic solar fuel generation. Energy Fuels 2021, 35, 18888–18899. doi:10.1021/acs.energyfuels.1c02675. [Google Scholar]
-
Ye K, Imran M, Chen X, Zhao J. Triplet Photosensitizers and Their Applications in Triplet–Triplet Annihilation Upconversion. ACS Appl. Opt. Mater. 2024, 2, 1803–1824. doi:10.1021/acsaom.4c00016. [Google Scholar]
-
Schloemer T, Narayanan P, Zhou Q, Belliveau E, Seitz M, Congreve DN. Nanoengineering triplet–triplet annihilation upconversion: From materials to real-world applications. ACS Nano 2023, 17, 3259–3288. doi:10.1021/acsnano.3c00543. [Google Scholar]
-
Wang Z, Zhao J, Barbon A, Toffoletti A, Liu Y, An Y, et al. Radical-Enhanced Intersystem Crossing in New Bodipy Derivatives and Application for Efficient Triplet-Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2017, 139, 7831–7842. doi:10.1021/jacs.7b02063. [Google Scholar]
-
Huang L, Wu W, Li Y, Huang K, Zeng L, Lin W, et al. Highly Effective Near-Infrared Activating Triplet-Triplet Annihilation Upconversion for Photoredox Catalysis. J. Am. Chem. Soc. 2020, 142, 18460–18470. doi:10.1021/jacs.0c06976. [Google Scholar]
-
Fan C, Wei L, Niu T, Rao M, Cheng G, Chruma JJ, et al. Efficient Triplet-Triplet Annihilation Upconversion with an Anti-Stokes Shift of 1.08 eV Achieved by Chemically Tuning Sensitizers. J. Am. Chem. Soc. 2019, 141, 15070–15077. doi:10.1021/jacs.9b05824. [Google Scholar]
-
Jin P, Xu X, Yan Y, Hammecke H, Wang C. Luminescent Fe(III) Complex Sensitizes Aerobic Photon Upconversion and Initiates Photocatalytic Radical Polymerization. J. Am. Chem. Soc. 2024, 146, 35390–35401. doi:10.1021/jacs.4c14248. [Google Scholar]
-
Huang Z, Tang ML. Designing Transmitter Ligands That Mediate Energy Transfer between Semiconductor Nanocrystals and Molecules. J. Am. Chem. Soc. 2017, 139, 9412–9418. doi:10.1021/jacs.6b08783. [Google Scholar]
-
Sun W, Ronchi A, Zhao T, Han J, Monguzzi A, Duan P. Highly efficient photon upconversion based on triplet–triplet annihilation from bichromophoric annihilators. J. Mater. Chem. C 2021, 9, 14201–14208. doi:10.1039/D1TC01569A. [Google Scholar]
-
Olesund A, Johnsson J, Edhborg F, Ghasemi S, Moth-Poulsen K, Albinsson B. Approaching the spin-statistical limit in visible-to-ultraviolet photon upconversion. J. Am. Chem. Soc. 2022, 144, 3706–3716. doi:10.1021/jacs.1c13222. [Google Scholar]
-
Cabanero DC, Rovis T. Low-energy photoredox catalysis. Nat. Rev. Chem. 2025, 9, 28–45. doi:10.1038/s41570-024-00663-6. [Google Scholar]
-
Ravetz BD, Pun AB, Churchill EM, Congreve DN, Rovis T, Campos LM. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. doi:10.1038/s41586-018-0835-2. [Google Scholar]
-
Huang L, Han G. Triplet-triplet annihilation photon upconversion-mediated photochemical reactions. Nat. Rev. Chem. 2024, 8, 238–255. doi:10.1038/s41570-024-00585-3. [Google Scholar]
-
Schulze TF, Czolk J, Cheng Y-Y, Fückel B, MacQueen RW, Khoury T, et al. Efficiency enhancement of organic and thin-film silicon solar cells with photochemical upconversion. J. Phys. Chem. C 2012, 116, 22794–22801. doi:10.1021/jp309636m. [Google Scholar]
-
Nattestad A, Cheng YY, MacQueen RW, Schulze TF, Thompson FW, Mozer AJ, et al. Dye-sensitized solar cell with integrated triplet–triplet annihilation upconversion system. J. Phys. Chem. Lett. 2013, 4, 2073–2078. doi:10.1021/jz401050u. [Google Scholar]
-
Beery D, Schmidt TW, Hanson K. Harnessing sunlight via molecular photon upconversion. ACS Appl. Mater. Interfaces 2021, 13, 32601–32605. doi:10.1021/acsami.1c08159. [Google Scholar]
-
Carrod AJ, Gray V, Börjesson K. Recent advances in triplet–triplet annihilation upconversion and singlet fission, towards solar energy applications. Energy Environ. Sci. 2022, 15, 4982–5016. doi:10.1039/D2EE01600A. [Google Scholar]
-
Schulze TF, Schmidt TW. Photochemical upconversion: present status and prospects for its application to solar energy conversion. Energy Environ. Sci. 2015, 8, 103–125. doi:10.1039/C4EE02481H. [Google Scholar]
-
Naimovičius L, Bharmoria P, Moth-Poulsen K. Triplet–triplet annihilation mediated photon upconversion solar energy systems. Mater. Chem. Front. 2023, 7, 2297–2315. doi:10.1039/D3QM00069A. [Google Scholar]
-
Dou Q, Jiang L, Kai D, Owh C, Loh XJ. Bioimaging and biodetection assisted with TTA-UC materials. Drug Discov. Today 2017, 22, 1400–1411. doi:10.1016/j.drudis.2017.04.003. [Google Scholar]
-
Lin W, Li J, Feng H, Qi F, Huang L. Recent Advances in Triplet–Triplet Annihilation Upconversion for Bioimaging and Biosensing. J. Anal. Test. 2023, 7, 327–344. doi:10.1007/s41664-023-00264-0. [Google Scholar]
-
Huang L, Kakadiaris E, Vaneckova T, Huang K, Vaculovicova M, Han G. Designing next generation of photon upconversion: Recent advances in organic triplet-triplet annihilation upconversion nanoparticles. Biomaterials 2019, 201, 77–86. doi:10.1016/j.biomaterials.2019.02.008. [Google Scholar]
-
Zhang B, Richards KD, Jones BE, Collins AR, Sanders R, Needham SR, et al. Ultra-Small Air-Stable Triplet-Triplet Annihilation Upconversion Nanoparticles for Anti-Stokes Time-Resolved Imaging. Angew. Chem. Int. Ed. 2023, 62, e202308602. doi:10.1002/anie.202308602. [Google Scholar]
-
Meir R, Hirschhorn T, Kim S, Fallon KJ, Churchill EM, Wu D, et al. Photon upconversion hydrogels for 3D optogenetics. Adv. Funct. Mater. 2021, 31, 2010907. doi:10.1002/adfm.202010907. [Google Scholar]
-
Uji M, Kondo J, Hara-Miyauchi C, Akimoto S, Haruki R, Sasaki Y, et al. In Vivo Optogenetics Based on Heavy Metal-Free Photon Upconversion Nanoparticles. Adv. Mater. 2024, 36, 2405509. doi:10.1002/adma.202405509. [Google Scholar]
-
Sasaki Y, Oshikawa M, Bharmoria P, Kouno H, Hayashi-Takagi A, Sato M, et al. Near-infrared optogenetic genome engineering based on photon-upconversion hydrogels. Angew. Chem. Int. Ed. 2019, 58, 17827–17833. doi:10.1002/anie.201911025. [Google Scholar]
-
Huang L, Zeng L, Chen Y, Yu N, Wang L, Huang K, et al. Long wavelength single photon like driven photolysis via triplet triplet annihilation. Nat. Commun. 2021, 12, 122. doi:10.1038/s41467-020-20326-6. [Google Scholar]
-
Long K, Lv W, Wang Z, Zhang Y, Chen K, Fan N, et al. Near-infrared light-triggered prodrug photolysis by one-step energy transfer. Nat. Commun. 2023, 14, 8112. doi:10.1038/s41467-023-43805-y. [Google Scholar]
-
Zeng L, Jiang LH, Li JY, Huang L, Chen Y, Yu N, et al. Metal-Free Far-Red Light-Driven Photolysis via Triplet Fusion to Enhance Checkpoint Blockade Immunotherapy. Angew. Chem. Int. Ed. 2023, 62, e202218341. doi:10.1002/anie.202218341. [Google Scholar]
-
Huang L, Zhao Y, Zhang H, Huang K, Yang J, Han G. Expanding anti-Stokes shifting in triplet–triplet annihilation upconversion for in vivo anticancer prodrug activation. Angew. Chem. Int. Ed. 2017, 129, 14592–14596. doi:10.1002/ange.201704430. [Google Scholar]
-
Borisov SM, Larndorfer C, Klimant I. Triplet-Triplet Annihilation-Based Anti-Stokes Oxygen Sensing Materials with a Very Broad Dynamic Range. Adv. Funct. Mater. 2012, 22, 4360–4368. doi:10.1002/adfm.201200794. [Google Scholar]
-
Xu M, Zou X, Su Q, Yuan W, Cao C, Wang Q, et al. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat. Commun. 2018, 9, 2698. doi:10.1038/s41467-018-05160-1. [Google Scholar]
-
Huang L, Le T, Huang K, Han G. Enzymatic enhancing of triplet-triplet annihilation upconversion by breaking oxygen quenching for background-free biological sensing. Nat. Commun. 2021, 12, 1898. doi:10.1038/s41467-021-22282-1. [Google Scholar]
-
Wang X, Ding F, Jia T, Li F, Ding X, Deng R, et al. Molecular near-infrared triplet-triplet annihilation upconversion with eigen oxygen immunity. Nat. Commun. 2024, 15, 2157. doi:10.1038/s41467-024-46541-z. [Google Scholar]
-
Feng HJ, Zeng L, Li JY, Lin WY, Qi F, Jiang LH, et al. Natural Protein Photon Upconversion Supramolecular Assemblies for Background-Free Biosensing. J. Am. Chem. Soc. 2024, 146, 21791–21805. doi:10.1021/jacs.4c06012. [Google Scholar]
-
Zhang MY, Feng HJ, Li JY, Jiang LH, Ma AX, Zeng L, et al. High-Performance 721 nm-Excitable Photon Upconversion Porous Aromatic Frameworks for Broad-Range Oxygen Sensing and Efficient Heterogeneous Photoredox Catalysis. Adv. Mater. 2025, 37, e2502150. doi:10.1002/adma.202502150. [Google Scholar]
-
Filatov MA, Baluschev S, Landfester K. Protection of densely populated excited triplet state ensembles against deactivation by molecular oxygen. Chem. Soc. Rev. 2016, 45, 4668–4689. doi:10.1039/C6CS00092D. [Google Scholar]
-
Huang Z, Tung C-H, Wu L-Z. Quantum Dot-Sensitized Triplet–Triplet Annihilation Photon Upconversion for Solar Energy Conversion and beyond. Acc. Mater. Res. 2024, 5, 136–145. doi:10.1021/accountsmr.3c00186. [Google Scholar]
-
Flors C, Griesbeck AG, Vassilikogiannakis G. Singlet Oxygen: Chemistry, Applications and Challenges Ahead. ChemPhotoChem 2018, 2, 510–511. doi:10.1002/cptc.201800120. [Google Scholar]
-
Fan X, Fu Q, Liu G, Jia H, Dong X, Li Y-F, et al. Applying molecular oxygen for organic pollutant degradation: Strategies, mechanisms, and perspectives. Environ. Sci. Ecotechnol. 2024, 22, 100469. doi:10.1016/j.ese.2024.100469. [Google Scholar]
-
Qi F, Feng H-J, Peng Y, Jiang L-H, Zeng L, Huang L. New Type Annihilator of π-Expanded Diketopyrrolopyrrole for Robust Photostable NIR-Excitable Triplet–Triplet Annihilation Upconversion. ACS Appl. Mater. Interfaces 2024, 16, 7512–7521. doi:10.1021/acsami.3c17679. [Google Scholar]
-
Liu Q, Xu M, Yang T, Tian B, Zhang X, Li F. Highly Photostable Near-IR-Excitation Upconversion Nanocapsules Based on Triplet–Triplet Annihilation for In Vivo Bioimaging Application. ACS Appl. Mater. Interfaces 2018, 10, 9883–9888. doi:10.1021/acsami.7b17929. [Google Scholar]
-
Fallon KJ, Churchill EM, Sanders SN, Shee J, Weber JL, Meir R, et al. Molecular Engineering of Chromophores to Enable Triplet–Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2020, 142, 19917–19925. doi:10.1021/jacs.0c06386. [Google Scholar]
-
Pun AB, Campos LM, Congreve DN. Tunable Emission from Triplet Fusion Upconversion in Diketopyrrolopyrroles. J. Am. Chem. Soc. 2019, 141, 3777–3781. doi:10.1021/jacs.8b11796. [Google Scholar]
-
Singh-Rachford TN, Haefele A, Ziessel R, Castellano FN. Boron Dipyrromethene Chromophores: Next Generation Triplet Acceptors/Annihilators for Low Power Upconversion Schemes. J. Am. Chem. Soc. 2008, 130, 16164–16165. doi:10.1021/ja807056a. [Google Scholar]
-
Jiang L-H, Zeng L, Zhang M-Y, Huang L, Pang D-W. Highly Effective Near-Infrared Light-Activatable Triplet Fusion Upconversion with BODIPY Annihilators: Triplet Modulation via Non-Conjugated n–π Spatial Electron Coupling. Adv. Opt. Mater. 2024, 12, 2301879. doi:10.1002/adom.202301879. [Google Scholar]
-
Liu Q, Yin B, Yang T, Yang Y, Shen Z, Yao P, et al. A General Strategy for Biocompatible, High-Effective Upconversion Nanocapsules Based on Triplet–Triplet Annihilation. J. Am. Chem. Soc. 2013, 135, 5029–5037. doi:10.1021/ja3104268. [Google Scholar]
-
Wan S, Lin J, Su H, Dai J, Lu W. Photochemically deoxygenating solvents for triplet-triplet annihilation photon upconversion operating in air. Chem. Commun. 2018, 54, 3907–3910. doi:10.1039/c8cc00780b. [Google Scholar]
-
Wan S, Lu W. Reversible Photoactivated Phosphorescence of Gold(I) Arylethynyl Complexes in Aerated DMSO Solutions and Gels. Angew. Chem. Int. Ed. Engl. 2017, 56, 1784–1788. doi:10.1002/anie.201610762. [Google Scholar]
-
Wei Y, Zheng M, Chen L, Zhou X, Liu S. Near-infrared to violet triplet-triplet annihilation fluorescence upconversion of Os(ii) complexes by strong spin-forbidden transition. Dalton Trans. 2019, 48, 11763–11771. doi:10.1039/c9dt02276g. [Google Scholar]
-
Feng H-J, Qi F, Li J-Y, Lin W-Y, Jiang L-H, Zhang M-Y, et al. Dual Roles of the Photo-oxidation of Organic Amines for Enhanced Triplet–Triplet Annihilation Upconversion in Nanoparticles. Nano Lett. 2024, 24, 8770–8777. doi:10.1021/acs.nanolett.4c02529. [Google Scholar]
-
Qi F, Feng H-J, Li J-Y, Peng Y, Jiang L-H, Li Y-Z, et al. Amino Acids-Enabled Fast-Restore of Triplet-Triplet Annihilation Upconversion Luminescence for Background-Free Sensing of Herbicides. Small Methods 2025, 9, 2401945. doi:10.1002/smtd.202401945. [Google Scholar]
-
Park J, Xu M, Li F, Zhou HC. 3D Long-Range Triplet Migration in a Water-Stable Metal-Organic Framework for Upconversion-Based Ultralow-Power in Vivo Imaging. J. Am. Chem. Soc. 2018, 140, 5493–5499. doi:10.1021/jacs.8b01613. [Google Scholar]
-
Vadrucci R, Monguzzi A, Saenz F, Wilts BD, Simon YC, Weder C. Nanodroplet-Containing Polymers for Efficient Low-Power Light Upconversion. Adv. Mater. 2017, 29, 1702992. doi:10.1002/adma.201702992. [Google Scholar]
-
Duan P, Yanai N, Nagatomi H, Kimizuka N. Photon upconversion in supramolecular gel matrixes: Spontaneous accumulation of light-harvesting donor-acceptor arrays in nanofibers and acquired air stability. J. Am. Chem. Soc. 2015, 137, 1887–1894. doi:10.1021/ja511061h. [Google Scholar]
-
Wei L, Fan C, Rao M, Gao F, He C, Sun Y, et al. Triplet-triplet annihilation upconversion in LAPONITE®/PVP nanocomposites: Absolute quantum yields of up to 23.8% in the solid state and application to anti-counterfeiting. Mater. Horiz. 2022, 9, 3048–3056. doi:10.1039/d2mh00887d. [Google Scholar]
-
Wei L, Yang C, Wu W. Optimizing TTA-UC performance by chemically tuning sensitizers and orderly organizing sensitizers and annihilators. Chem. Commun. 2025, 61, 7221–7235. doi:10.1039/d5cc00476d. [Google Scholar]
-
Watanabe S, Mizukami K, Kimizuka N, Yasuda T. Visible-to-UV photon upconversion in metal-free molecular aggregates based on glassy diphenylnaphthalene derivatives. J. Mater. Chem. C 2024, 12, 10874–10878. doi:10.1039/d4tc01820f. [Google Scholar]
-
Bharmoria P, Hisamitsu S, Sasaki Y, Kang TS, Morikawa M-A, Joarder B, et al. Photon upconverting bioplastics with high efficiency and in-air durability. J. Mater. Chem. C 2021, 9, 11655–11661. doi:10.1039/d1tc00287b. [Google Scholar]
-
Bharmoria P, Edhborg F, Bildirir H, Sasaki Y, Ghasemi S, Martensson A, et al. Recyclable optical bioplastics platform for solid state red light harvesting via triplet-triplet annihilation photon upconversion. J. Mater. Chem. A 2022, 10, 21279–21290. doi:10.1039/d2ta04810h. [Google Scholar]
-
Wei Y, Li Y, Li Z, Xu X, Cao X, Zhou X, et al. Efficient Triplet-Triplet Annihilation Upconversion in Solution and Hydrogel Enabled by an S-T Absorption Os(II) Complex Dyad with an Elongated Triplet Lifetime. Inorg. Chem. 2021, 60, 19001–19008. doi:10.1021/acs.inorgchem.1c02846. [Google Scholar]
-
Oddo AM, Mani T, Kumar CV. Micelles Embedded in Multiphasic Protein Hydrogel Enable Efficient and Air-Tolerant Triplet Fusion Upconversion with Heavy-Atom and Spin-Orbit Charge-Transfer Sensitizers. Acs Appl. Mater. Interfaces 2020, 12, 39293–39303. doi:10.1021/acsami.0c11202. [Google Scholar]
-
Enomoto R, Hoshi M, Oyama H, Agata H, Kurokawa S, Kuma H, et al. van der Waals solid solution crystals for highly efficient in-air photon upconversion under subsolar irradiance. Mater. Horiz. 2021, 8, 3449–3456. doi:10.1039/d1mh01542g. [Google Scholar]
-
Fukuuchi R, Toyoshima Y, Yoshinami T, Tripathi N, Heck C, Kobayashi K, et al. Solution to the Host-Guest Compatibility Problem of Solid Triplet-Triplet Annihilation Photon Upconversion by a Molecular-Anchor Sensitizer Approach. J. Phys. Chem. C 2024, 128, 2604–2617. doi:10.1021/acs.jpcc.3c06608. [Google Scholar]
-
Enomoto R, Murakami Y. Solvent-free temperature gradient melt formation of efficient visible-to-UV photon upconversion organic films with subsolar threshold and over 100 h photostability in air. J. Mater. Chem. C 2023, 11, 1678–1683. doi:10.1039/d2tc04578h. [Google Scholar]
-
Enomoto R, Murakami Y. Absorption bandwidth broadening of photon upconversion solid-solution organic crystals by co-dissolution of multiple sensitizers. Appl. Phys. Express 2023, 16, 092001. doi:10.35848/1882-0786/acf6a7. [Google Scholar]
-
Liang W, Nie C, Du J, Han Y, Zhao G, Yang F, et al. Near-infrared photon upconversion and solar synthesis using lead-free nanocrystals. Nat. Photonics 2023, 17, 346–353. doi:10.1038/s41566-023-01156-6. [Google Scholar]