1. Introduction
Excessive friction and wear contribute substantially to energy losses and environmental contamination. With the developing global need for energy and the growing environmental concerns. A new generation of lubricants, characterized by biodegradability, high energy efficiency, and environmental compatibility, is on the horizon. As a type of biodegradable lubricant, long-chain fatty acid esters have been widely used in industry due to their characteristics such as high viscosity index, thermal stability, and biodegradability [
1,
2,
3,
4]. The traditional method of synthesizing pentaerythrotol fatty acid esters is to esterify pentaerythrotol with fatty acids. The esterification reaction can proceed without a catalyst, but the reaction rate is slow. The catalyst is essential for driving the esterification reaction: The sulfuric acid, solid acid catalyst, brönsted acid ionic liquid have been studied since the 1990s [
5,
6,
7]. However, these catalysts always need to be separated from the products, which brings great inconvenience to industrial production for ester oils. Therefore, it is urgent to develop a new type of material that does not require separation after esterification and then acts as a lubricant additive, which is one of the effective ways to solve the problem of separating the catalyst from ester oil products.
Ionic liquids have attracted considerable interest in recent years due to their unique physicochemical properties, such as non-volatile, non-flammable and explosive, relatively high chemical and thermodynamic stability,
etc. In addition, ionic liquids also have a wide range of characteristics such as a broad liquid temperature range, good solubility for organic and inorganic compounds, a wide electrochemical window, designable structure, and tunable properties [
8,
9,
10]. Ionic liquids have shown good catalytic performance in the field of catalysis: esterification [
11,
12,
13], transesterification [
14,
15], esterification of phosphinic acids, phosphonic acids and phosphoric acid monoesters [
16,
17] included. In recent years, ionic liquids have emerged as promising alternatives, serving as a catalyst favored by the majority of scientific researchers due to their performance of environmentally friendly. Multiple brönsted acidic ionic liquids are catalysts for esterification reactions, with high catalytic activity and easy separation [
18,
19,
20]. Actually, the lubricating performance of ionic liquids has also attracted much attention: ionic liquids, as a lubricant, have a wide temperature range and extremely low vapor pressure, endowing them with excellent comprehensive lubrication performance unmatched by other lubricating materials. For example, ionic liquids can solve problems such as easy solidification and volatilization of lubricants under harsh conditions [
21,
22,
23,
24,
25,
26].
The introduction of two or more functional groups into an ionic liquid can regulate the multifunctionalization of the ionic liquid, to construct a functional ionic liquid with a novel structure and more prominent advantages. A series of functionalized ionic liquids were prepared as green reaction media and catalysts [
27,
28,
29], however, up to now, there have been no reports on the research and application of bi-funtionalized ionic liquids as catalysts for the synthesis of ester oils, and can also play the role of lubricant additives in the synthesized ester oil. In this paper, the bi-functionalized ionic liquids were prepared, and the chain length of the substituent of the ionic liquid can be reasonably controlled to realize the bi-functionalized ionic liquid coordination and optimization of catalytic performance and lubrication performance. Meanwhile, the problem of separating the catalyst from ester oil products was solved.
2. Materials and Methods
All chemicals were obtained commercially without further purification. The purity and manufacturer are listed in the Supplementary Materials Table S1.
2.1. Ionic Liquid Preparation
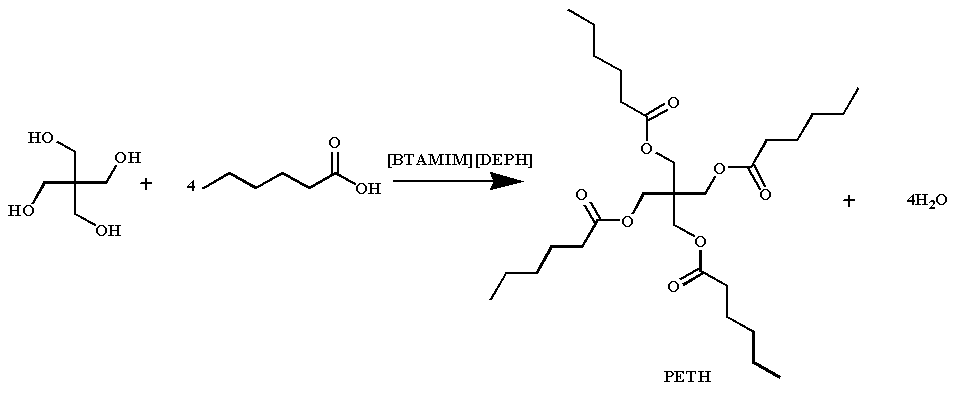
120 mL dichloromethane and 5 mL deionized water were added to a 250 mL round bottom flask. A certain amount of 1-alkyl-3-methylimidazolium bromide and bis(2-ethylhexyl) hydrogen phosphate was introduced and stirred at room temperature. A 30% wt sodium hydroxide solution was dropped into the bottom flask and stirred for 2 h at 25 ℃. The products were washed with deionized water 6 times and then evaporated to remove water and dichloromethane. After that, a bi-functionalized ionic liquid 1-alkyl-3-methylimidazolium bis(2-ethylhexyl)hydrogen phosphate was obtained by vacuum drying at 70 ℃. 1-butyl-3-methylimidazolium bis(2-ethylhexyl) phosphate was labeled as [BMIM][DEHP]; 1-hexyl-3-methylimidazolium bis(2-ethylhexyl) phosphate was labeled as [HMIM][DEHP]; 1-octyl-3-methylimidazolium bis(2-ethylhexyl) phosphate was labeled as [OMIM][DEHP]; 1-decyl-3-methylimidazolium bis(2-ethylhexyl) phosphate was labeled as [DMIM][DEHP].
2.2. Catalytic Performance Test of Ionic Liquids
Using the esterification reaction of pentaerythritol and hexanoic acid in a stoichiometric ratio as a model reaction, magnetic stirring was performed in a 100 mL three-necked round bottom flask. 0.013 mol of pentaerythrotol, 0.052 mol of hexanoic acid, and 0.16 mmol of catalyst were added, along with 2 mL of toluene as a water scavenger. The reaction was carried out at 165 °C for a specified time. The product was purified by rotary evaporation. The acid value of the product was determined according to ISO 6618: 1997(E) [
30], and the degree of esterification of the product was analyzed based on the acid value. The following formula can calculate the esterification rate:
In the formula, Er represents the esterification reaction of pentaerythrotol and hexanoic acid, AN
a is the acid value of the product after the reaction, ANb is the acid value of the substrate before the reaction. R denotes the molar ratio of hexanoic acid to pentaerythrotol.
2.3. Tribological Performance Test of [HMIM][DEHP]
The tribological properties were tested by an optimmol SRV tester under the condition of load 50 N, frequency 50 Hz, temperature 50 ℃, stroke 1 mm, time 30 min. The upper ball was AISI52100 steel, with a diameter of 10 mm and a rock-well hardness of 58–62 HRC. The lower disc was AISI52100 steel with a rock-well hardness of 60–64 HRC, and the diameter of the disc is 24 mm. The COF was recorded automatically.
3. Results and Discussion
3.1. Catalytic Properties
The esterification reaction of pentaerythritol with hexanoic acid was selected as the model reaction for the study of ionic liquid catalytic performance. The influence of the carbon chain length of 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphates and the reaction time was investigated, and the results are shown in . Comparing the esterification of the pentaerythrotol with hexanoic acid by 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphates with different length of carbon chain in imidazoline cation, the 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphate with 4 carbons in imidazoline cation exhibited the best catalytic activity. Meanwhile, the ionic liquid with 6 carbons in the imidazoline cation also showed a good catalytic activity. Since the acidity of 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphate decreases with the carbon chain length in imidazoline cation, we select [HMIM][DEHP] to further examine the effect of reaction time on the activity of pentaerythrotol esterification with hexanoic acid catalyst. It can be seen that the esterification was up to 99% when the reaction time was 7 h. Therefore, all 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphates had catalytic properties, and the [HMIM][DEHP] was the optimum one.
.
Catalytic performance of 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphates for the esterification a.
 |
| Entry |
Catalyst |
Time/h |
AN/(mg KOH/g) |
Er/% |
| 1 |
none |
1 |
306 |
29 |
| 2 |
[BMIM][DEHP] |
1 |
131 |
70 |
| 3 |
[HMIM][DEHP] |
1 |
139 |
68 |
| 4 |
[OMIM][DEHP] |
1 |
165 |
62 |
| 5 |
[DMIM][DEHP] |
1 |
187 |
56 |
| 6 |
[HMIM][DEHP] |
3 |
49 |
89 |
| 7 |
[HMIM][DEHP] |
5 |
32 |
93 |
| 8 b |
[HMIM][DEHP] |
7 |
2 |
99 |
3.2. Tribological Properties
shows the variation of the friction coefficients of pentaerythrotol tetra-hexanoate and the pentaerythrotol tetra-hexanoate plus [HMIM][DEHP]. The friction coefficient of the pentaerythrotol tetra-hexanoate with [HMIM][DEHP] as catalyst and additive decreased, which shows that pentaerythrotol tetra-hexanoate with [HMIM][DEHP] as catalyst and additive had better friction-reducing properties than the pentaerythrotol tetra-hexanoate. The result confirms that the [HMIM][DEHP] could improve the tribological properties of pentaerythrotol tetra-hexanoate.
. Variation of friction coefficient with time for PETH and PETH + [HMIM][DEHP].
The wear scar diameter of the upper running ball was tested by an optical microscope (OLYMPUS BX41, OlympusCorporation, Tokyo, Japan), and the images are shown in , and the lists the specific data. The wear scar diameter of the upper running ball lubricated with pentaerythrotol tetra-hexanoate is higher than that lubricated with pentaerythrotol tetra-hexanoate with [HMIM][DEHP] as catalyst and lubricant additive, and the result indicates that the introduction of [HMIM][DEHP] could improve the anti-wear performance of pentaerythrotol tetra-hexanoate. This is consistent with the result of SRV friction test.
.
The specific value of wear scar diameter of the upper running ball lubricated by PETH and PETH+[HMIM][DEHP] a.
| Lubricant |
PETH |
PETH + [HMIM][DEHP] |
| WSD (wear scar diameter)/mm |
0.59 |
0.47 |
. Lubricated ball wear scar diameter (<b>a</b>) PETH, (<b>b</b>) PETH + [HMIM][DEHP].
3.3.1. The Stability of [HMIM][DEHP]
The TG/DTG analysis of [HMIM][DEHP] was conducted, with the results shown in . The decomposition of [HMIM][DEHP] commenced at approximately 261.1 ℃ and was completed at 429.9 ℃. Simultaneously, the esterification reaction was carried out at 165 °C, which is significantly lower than the initial decomposition temperature of [HMIM][DEHP]. Thus, [HMIM][DEHP] exhibited excellent stability during the reaction process.
. The TG/DTG characterizations of [HMIM][DEHP].
The XPS spectra of N1s and P2p for [HMIM][DEHP] and [HMIM][DEHP] + PETH (the product without catalyst separation) are shown in . It can be observed that the binding energies of N1s and P2p for [HMIM][DEHP] are consistent with those for [HMIM][DEHP] + PETH. The results indicate that [HMIM][DEHP] remains stable during the esterification process, as confirmed by TG/DTG analysis.
. The XPS spectra of N1s and P2p in (a) [HMIM][DEHP], (b) PETH + [HMIM][DEHP].
The Fourier Transform Infrared Spectroscopy (FT-IR) spectra are shown in . As can be known from , the peak at 1150 cm
−1 belongs to the P-O-C stretching vibration, and the peak at 1360 cm
−1 belongs to the P=O stretching vibration [
31]. The positions of the two adsorption peaks of [HMIM][DEHP] have not changed after the reaction, illustrating that the structure of [HMIM][DEHP] is stable. This result verifies the conclusions of TG and XPS.
. The FT-IR spectra of (a) [HMIM][DEHP] and (b) PETH + [HMIM][DEHP].
3.3.2. The Worn Surface Analysis
The worn surface morphology of the lower steel disc lubricated with PETH and PETH + [HMIM][DEHP] was characterized using scanning electron microscopy (SEM, model JSM-6701F, Japan Electron Optics Laboratory, Tokyo, Japan). The results are presented in . It was observed that the wear scar on the disc lubricated with PETH alone was significantly wider and deeper than that on the disc lubricated with PETH + [HMIM][DEHP]. In contrast, the wear scar on the disc lubricated with the PETH + [HMIM][DEHP] combination was relatively narrow and exhibited a smoother surface morphology. These findings are consistent with the optical microscopy observations of the wear on the upper running ball, further supporting the improved lubricating performance of PETH in the presence [HMIM][DEHP].
. The SEM images of the worn surfaces lubricated with (<b>a</b>,<b>b</b>) PETH, (<b>c</b>,<b>d</b>) PETH + [HMIM][DEHP].
Wear volume and Surface profiles of wear surfaces after the friction test were characterized by a KLA-TENCOR MicroXAM-800 white light interferometry three-dimensional profiler, and the images were presented in and . As presented in , compared with the base oil PETH, introducing [HMIM][DEHP] reduces the wear volume of the lubricants, which means an improvement in the anti-wear performance of the lubricants. The reduction in wear volume can reach up to 46.8% when the ionic liquid [HMIM][DEHP] content is only 1% (molar ratio). As shown in , the wear tracks for PETH + [HMIM][DEHP] are notably narrower and shallower than those for the base oil PETH, which is consistent with the wear volume data and further supports the friction-reducing properties indicated by the friction coefficient.
. The wear volume of wear surfaces after the friction test.
. Surface profiles of wear surfaces after friction test (<b>a</b>,<b>b</b>) Base oil PETH, (<b>c</b>,<b>d</b>) PETH + [HMIM][DEHP].
To further investigate the lubrication mechanism of [HMIM][DEHP], XPS analysis was performed to examine the chemical states of Fe2p, N1s, P2p, and O1s on the worn surface lubricated with PETH + [HMIM][DEHP]. The corresponding spectra are presented in
. Significant changes were observed in the N1s, P2p, and O1s spectra from the worn surface compared to those of pure [HMIM][DEHP] and pure PETH, indicating that complex tribochemical reactions occurred during the friction process. The Fe2p spectra exhibited peaks at approximately 707.1 eV, 710.3 eV, 719.2 eV, and 724.3 eV, which can be attributed to FeP, Fe₂P, FeO, Fe₃O₄, and Fe
2O
3 [
32,
33,
34]. The P2p signal at 133.70 eV is assigned to FePO
4, while the O1s peaks around 531.9 eV and 530.1 eV correspond to FeO, Fe
2O
3, and Fe
3O
4 [
32,
35,
36]. Additionally, the N1s peak at 402.6 eV may be associated with oxynitride or nitrogen-nitrogen double bonds. The combined effects of high pressure, heat, catalytic activity, and mechanical energy during friction induced these tribochemical reactions, forming compounds such as FeP, Fe₂P, FeO, Fe
3O
4, Fe
2O
3, and FePO
4. These products suggest the development of a boundary lubrication film in the contact zone during the SRV test. As a result, the friction-reducing and anti-wear properties were significantly improved.
. The XPS spectra of (a) pure PETH, (b) pure [HMIM][DEHP] and (c) worn surface lubricated with PETH + [HMIM][DEHP].
4. Conclusions
A series of bi-functional ionic liquids 1-alkyl-3-methylimidazolium bis(2-ethylhexyl) phosphate were prepared: 1-butyl-3-methylimidazole bis(2-ethylhexyl) phosphate, 1-hexyl-3-methylimidazole bis(2-ethylhexyl) phosphate, 1-octyl-3-methylimidazole bis(2-ethylhexyl) phosphate, 1-decyl-3-methylimidazole bis(2-ethylhexyl) phosphate, and the 1-hexyl-3-methylimidazole bis(2-ethylhexyl) phosphate ([HMIM][DEHP]) was chosen to investigate the catalytic and tribological properties. The conclusions were as follows:
The catalytic properties of 1-hexyl-3-methylimidazole bis(2-ethylhexyl) phosphate were examined by the esterification of pentaerythrotol with hexanoic acid at a stoichiometric ratio. The conversion of hexanoic acid is up to 99% under mild conditions, and the ionic liquid catalyst does not need to be separated after the reaction, which can solve the scientific and technical problems of the widespread negative impact of residual catalyst on synthetic ester oil. The preparation procedure of the synthetic ester is shortened, which is conducive to industrial production and promotes its wide application.
The coefficient of friction and the wear scar of the up running ball lubricated by pentaerythrotol tetra-hexanoate an pentaerythrotol tetra-hexanoate plus [HMIM][DEHP] indicated that the [HMIM][DEHP] had good friction-reducing and anti-wear properties. The ionic liquid catalyst does not need to be separated after the reaction and plays the role of a lubricant additive in the ester oil. It not only helps to improve the performance of ester lubricating oil significantly, but also gives full play to the synergistic lubrication effect of synthetic ester lubricating oil and ionic liquid.
Supplementary Materials
The following supporting information can be found at: https://www.sciepublish.com/article/pii/663.
Figure S1: The
1H NMR spectra of [HMIM][DEHP];
Figure S2: The
13C NMR spectra of [HMIM][DEHP];
Figure S3: The
1H NMR spectra of PETH + [HMIM][DEHP].
Table S1: The vendors and quality or grade of chemicals.
Acknowledgments
We express our thanks for funding support from the Smart Grid-National Science and Technology Major Project (grant No. 2024ZD0803200).
Author Contributions
Experimental designing, data collection and analysis, writing manuscript, Y.W.; supervision, H.S. and C.J.; formal analysis, Q.Z. (Qin Zhao); investigation, Q.Z. (Qilong Zhao); editing, W.L.; conceptualization, Q.J. All authors have read and agreed to the published version of the manuscript.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Funding
This work is supported by the Smart Grid-National Science and Technology Major Project (grant No. 2024ZD0803200).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1.
Correa APL, Bastos RRC, Filho GNR, Zamian JR, Conceição LRV. Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction.
RSC Adv. 2020,
10, 20245–20256.
[Google Scholar]
2.
Jiang C, Lu Y, Li Z, Li C, Yan R. Enzymatic synthesis of L-ascorbyl fatty acid esters under ultrasonic irradiation and comparison of their antioxidant activity and stability.
Jounal Food Sci. 2016,
81, C1370.
[Google Scholar]
3.
Olusegun A, Adel P. Preparation of starch-fatty acid esters from agro-based raw material (corn starch, cassava starch, coconut oil and castor seed oil).
Starch Stärke 2023,
75, 2200205.
[Google Scholar]
4.
Wedler G, Trusler JPM. Review of density and viscosity data of pure fatty acid methyl ester, ethyl ester and butyl ester.
Fuel 2023,
339, 127466.
[Google Scholar]
5.
Nowak P. Kinetics of the liquid phase esterification of acrylic acid with n-octanol and 2-ethylhexanol catalyzed by sulfuric acid.
React. Kinet. Catal. Lett. 1999,
66, 375–380.
[Google Scholar]
6.
Ding J, He B, Tu J, Li J. Heat-activated zirconium sulfate as acid heterogeneous catalyst for biodiesel production.
J. Biobased Mater. Bioenergy 2012,
6, 142–147.
[Google Scholar]
7.
Zhang L, Xian M, He Y, Li L, Yang J, Yu S, et al. A br¢nsted acidic ionic liquid as an efficient and environmentally benign catalyst for biodiesel syn thesis from free fatty acids and alcohols.
Bioresour. Technol. 2009,
100, 4368–4373.
[Google Scholar]
8.
Tereshatov EE, Boltoeva M, Mazan V, Baley C, Folden CM, III. Hydrophobic polymerized ionic liquids for trace metal solid phase extraction: thallium transfer from hydrochloric acid media.
New J. Chemstry 2019,
43, 8958–8969.
[Google Scholar]
9.
Chen Y, Yu D, Liu Z, Xue Z, Mu T. Thermal, chemical, electrochemical, radiolytic and biological stability of ionic liquids and deep eutectic solvents.
New J. Chemstry 2022,
46, 17640–17668.
[Google Scholar]
10.
Avilés MD, Ramón P, Sanes J, Carrión FJ, Bermúdez MD. Fatty Acid-Derived Ionic Liquid Lubricant. Protic Ionic Liquid Crystals as Protic Ionic Liquid Additives.
Coatings 2019,
9, 710.
[Google Scholar]
11.
Zeng Q, Song Z, Qin H, Cheng H, Chen L, Pan M, et al. Ionic liquid [BMIm][HSO
4] as dual catalyst-solvent for the esterification of hexanoic acid with n-butanol.
Catal. Today 2020,
339, 113–119.
[Google Scholar]
12.
Bian Y, Liu J, Deng S, Zhang G, Peng Z, Li C. Ionic liquid functionalized polymer as an efficient solid acid catalyst for the esterification between methanol and methylacrylic acid.
Catal. Lett. 2024,
154, 2112–2123.
[Google Scholar]
13.
Shahbazi M, Tavakoli A, Hosseini M, Khanian M. 2-Hydroxyethylammonium bisulfate (2-HEAS) as bronsted acidic ionic liquid catalyst for esterification.
Ind. Eng. Chem. Res. 2022,
61, 7874–7880.
[Google Scholar]
14.
Zhang Y, Liu Y, Yang Z, Pan Z, Qiao C. Alkaline ILs supported onto mesoporous polymeric 3D spheres for catalytic synthesizing dimethyl carbonate in transesterification.
ChemistrySelect 2024,
9, e202401921.
[Google Scholar]
15.
Fuster MG, Moulefera I, Montalban MG, Villora G. Biocatalytic transesterification of salmon oil in ionic liquid media to obtain concentrations of omega-3 polyunsaturated fatty acids.
Eur. Food Res. Technol. 2024,
250, 1707–1719.
[Google Scholar]
16.
Kiss NZ, Keglevich G. Microwave -assisted direct esterification of cyclic phosphinic acids in the presence of ionic liquids.
Tetrahedron Lett. 2016,
57, 971–974.
[Google Scholar]
17.
Kiss NZ, Keglevich G. Direct esterification of phosphinic and phosphonic acids enhanced by ionic liquid additives.
Pure Appl. Chem. 2019,
91, 59–65.
[Google Scholar]
18.
Shi H, Zhu W, Li H, Liu H, Zhang M, Yan Y, et al. Microwave-accelerated esterification of salicylic acid using Brönsted acidic ionic liquids as catalysts.
Catal. Commun. 2010,
11, 588–591.
[Google Scholar]
19.
Thul M, Pantawane A, Lin W, Lin Y, Su P, Tseng S, et al. Tunable aryl imidazolium ionic liquids (TAIILs) as environmentally benign catalysts for the esterification of fatty acids to biodiesel fuel.
Catal. Commun. 2021,
149, 106243.
[Google Scholar]
20.
Xu Z, Zhao G, Ullah L, Wang M, Wang A, Zhang Y, et al. Acidic ionic liquid based UiO-67 type MOFs: a stable and efficient heterogeneous catalyst for esterification.
RSC Adv. 2018,
8, 10009–10016.
[Google Scholar]
21.
Cigno E, Magagnoli C, Pierce MS, Iglesias P. Lubricating ability of two phosphonium-based ionic liquids as additives of a bio-oil for use in wind turbines gearboxes.
Wear 2017,
376–377, 756–765.
[Google Scholar]
22.
Zhang Y, Cai T, Shang W, Sun L, Liu D, Tong D. Environmental friendly polyisobutylene-based ionic liquid containing chelated orthoborate as lubricant additive: Synthesis, tribological properties and synergistic interactions with ZDDP in hydrocarbon oils.
Tribol. Int. 2017,
115, 297–306.
[Google Scholar]
23.
Guo H, Adukure AR, Iglesias P. Effect of ionicity of three protic ionic liquids as neat lubricants and lubricant additives to a biolubricant.
Coatings 2019,
9, 713.
[Google Scholar]
24.
Buzolic JJ, Li H, Aman ZM, Silvester DS, Atkin R. Surface-active ionic liquids as lubricant additives to hexadecane and diethyl succinate.
Colloids Surf. A Physicochem. Eng. Asp. 2024,
699, 134669.
[Google Scholar]
25.
Sun Y, Qiu X, Liu Y, Sun S, Zhang C, Wang X, et al. A comparative study of the tribological performance of two oil-soluble ionic liquids as replacements for ZDDP (T204) additives in lubricants.
Tribol. Int. 2024,
198, 109843.
[Google Scholar]
26.
Waheed S, Ahmed A, Abid M, Mufti RA, Ferreira F, Bashir MN, et al. Ionic liquids as lubricants: An overview of recent developments.
J. Mol. Struct. 2024,
1301, 137307.
[Google Scholar]
27.
Wang P, Qiu J, Gao P, Dong R, Han Y, Fan M. The tribological behaviors and anti-corrosion performances of 5-phenylte trazole ionic liquid additives for water lubricants.
Wear 2023,
516–517, 204621.
[Google Scholar]
28.
Qiao Y, Chen L, Feiyan Y, Ding Z, Chen Z, Wang B, et al. Imidazolium ionic liquids as neat lubricant for electroplating Sn on Cu substrate under sliding electrical contact at high temperatures up to 80 ℃.
Appl. Surf. Sci. 2024,
643, 158677.
[Google Scholar]
29.
Shi F, Gu Y, Zhang Q, Deng Y. Development of ionic liquids as green reaction media and catalysts.
Catal. Surv. Asia 2004,
8, 179–186.
[Google Scholar]
30.
ISO 6618-1999(E); Ptroleum Products and Lubricants—Determination of Acid or Base Number—Colour-Indicator Titration Method; Technical Committee ISO/TC 28: Delft, The Netherlands, 1999.
31.
Liu Q, He H, Chao ZS, Xie J, Ruchenstein E. Synthesis of mesoporous chromium phosphates via solid-state reaction at low temperature.
New J. Chem. 2012,
36, 139–147.
[Google Scholar]
32.
Allen GC, Curtis MT, Hopper AJ, Tucker PM. X-ray photoelectron spectroscopy of iron-oxygen systems.
J. Chem. Soc. -Dalton Trans. 1974,
14, 1525–1530.
[Google Scholar]
33.
Myers CE, Franzen HF, Anderegg JW. X-ray photoelectron spectra and bonding in trasition-metal phosphides.
Inorg. Chem. 1985,
24, 1822–1824.
[Google Scholar]
34.
Tan BJ, Klabunde KJ, Sherwood PMA. X-ray photoelctron spectroscopy studies of solvented metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina.
Chem. Mater. 1990,
2, 186–191.
[Google Scholar]
35.
Lee YS, Kim HT, Yoo KO. Effect of ferric ixide on the high temperature removal of hydrogen sulfide over ZnO-Fe
2O
3 mixed metal oxide sorbent.
Ind. Eng. Chem. Res. 1995,
34, 1181–1188.
[Google Scholar]
36.
Barbaux Y, Dekiouk M, Maguer DL, Gengembre L, Huchette D, Grimblot J. Bulk and surface analysis of a Fe-P-O oxydehydrogenation catalyst.
Appl. Catal. A Gen. 1992,
90, 51–60.
[Google Scholar]
 Qin Zhao
1,2
Qilong Zhao
1,3
Cheng Jiang
1,2
Huaigang Su
1,2
Wenjing Lou
1,2,*
Qian Jia
1,2
Qin Zhao
1,2
Qilong Zhao
1,3
Cheng Jiang
1,2
Huaigang Su
1,2
Wenjing Lou
1,2,*
Qian Jia
1,2

