Recent Progress in Organically Modified Silica and Self-Matting Polymers for Coating Applications
Received: 05 November 2025 Revised: 12 December 2025 Accepted: 04 January 2026 Published: 09 January 2026
© 2026 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Coatings with high gloss have attracted people’s attention because of their radiant finish. However, as living standards rise, some problems associated with glossy and bright coatings have become more apparent. Firstly, reflection from high gloss coatings can cause discomfort to human eye. Second, minor imperfections such as dust accumulation, grease buildup, fingerprints, and small scratches become easily noticeable on the glossy surface, making coating appear unpleasant. These considerations created a need for low-gloss coating in surface ornamentation. Lastly, applying matte coatings to materials such as leather, paper, and fabrics can impart a soft, aesthetically pleasing, and natural appearance with comfortable tactility, lowering the plastic like sense produced by dazzling surfaces [1]. The ability of a coating to reflect light is indicated by the gloss, which describes an optical characteristic of a finished surface. A high gloss coating suggests that a large portion of incoming light is being reflected, making the surface glow. In contrast, the lower gloss indicates a lesser amount of light is reflected, resulting in a better quality of decorative surface appearance. The gloss of the coating is inversely proportional to its surface roughness; it decreases with increasing roughness of the coating film [2].
Common application areas of matting agents include decorative coatings, wood coatings, industrial coatings, and leather products, where consumers expect reduced reflection and improved visual focus. In decorative coatings, a matte finish is required, such as in theatres, because light reflecting off the walls can distract the audience and prevent full concentration on the screen. Hospitals also require matte-finish coatings to avoid distractions caused by light reflections [3]. In wood coatings, a matte finish enhances the appearance of wooden furniture and other wooden surfaces. In industrial coatings, a matte finish is applied to achieve an aesthetic appearance on machinery, equipment, and metal surfaces. In leather coatings, matting agents create a silky matte effect on leather goods, improving their visual appearance. Additionally, they enhance resistance to soiling, abrasion, and the wet look.
To achieve an effective matte effect in coatings, matting agents are required to meet various basic requirements including easy dispersibility, good suspension behaviors in liquid coating systems, consistent quality, and chemical inertness. Two types of matting agents reported in the literature: first, those based on insoluble particles, and second, those that create matting due to incompatibility with the paint system [4,5]. To achieve a uniform matting effect using insoluble particles, the particle size distribution should be as narrow as possible [6]. Currently, silica based matting agents are mostly used in coatings to create matte finish [7]. The silica based matting agent market has promising business opportunities in the coating due to the rising demand for matte finishes in coatings and paints. Growth in matte finish demand is mainly observed in the automotive and construction industries. The global market for nanoscale silica matting agents is projected to grow by 7.6% from 2024 to 2030 [8,9].
To determine effectiveness in matting, particle volume concentration (PVC) is the most important parameter for selecting the matting agent. Higher PVC gives a more micro-roughened surface and has less shine. Matting efficiency can be achieved either by adding more matting agent or selecting a grade with a larger pore capacity. The larger the pore volume, the less dense the particles are, resulting in more particles per unit mass. Particle size distribution is another important parameter; larger surface roughness is more likely to be altered by bigger particles for a given film thickness. However, excessive larger particles may lead to palpable roughness, which is normally undesirable. When matting agents are used, incompatibility between the additives and the polymer may lead to problems such as lacking light transmission, having an uneven gloss, or even forming a foggy surface. Furthermore, coated surfaces may result in brittle, powdery, or heavily seeded [10]. Additionally, there are various environmental concerns associated with conventional matting agents such as volatile organic compound (VOC). Mostly solvents are being used to disperse silica or waxes effectively, which increases VOC emissions and creates air pollution [11]. Fine silica particles released during the manufacturing and application process of traditional matting agents contributes to particulate matter pollution, raising occupational health and environmental safety concerns [12]. Lopez et al. reviewed literatures on efforts of the coating industry to reduce VOC [13].
To reduce these problems associated with silica, various surface modifications are needed for easy dispersibility. The best way to achieve compatibility is to modify inorganic matting agents with organic molecules, such as silane-modified silica, wax-treated silica, and polymeric matting agents. The primary advantage of these modifications is enhanced compatibility with different types of solvent-based and water-based coatings. PPG has developed a flexible surface treatment process for silica. The synthetic conditions used during precipitation can introduce reactive moieties into the batch, modifying the silica surface [14]. Young et al. reviewed polymeric matting agents with focus on ultraviolet (UV) coatings, powder coatings, and aqueous coatings [15]. This review comprises details on the matting mechanism, conventional matting agents, organically modified matting agents, and recent developments in polymer-based matting agents.
2. Mechanism of Matte Appearance
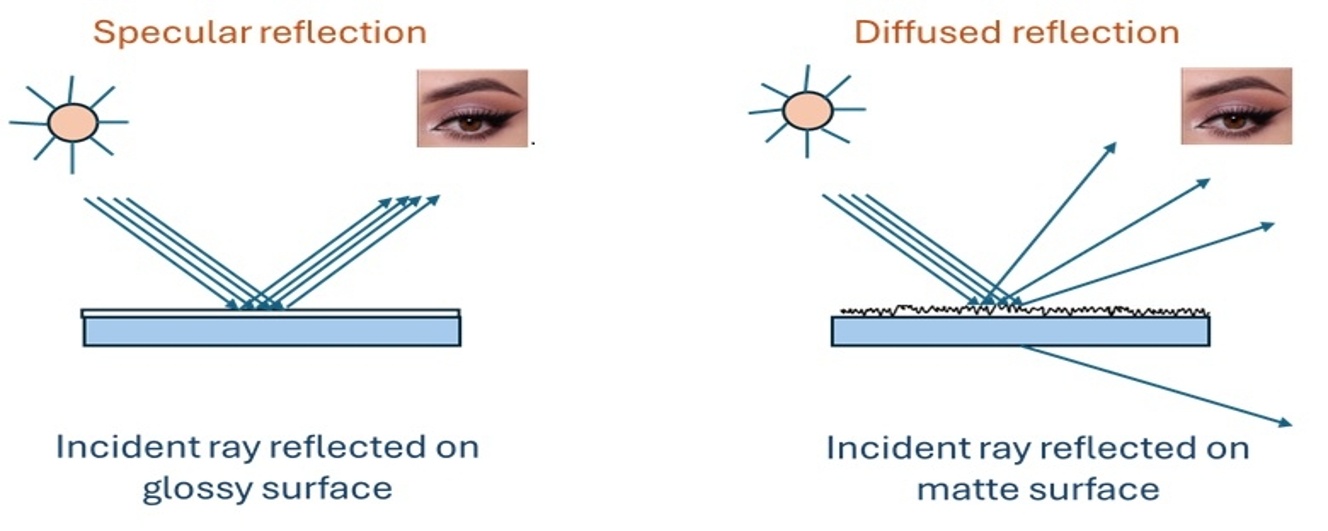
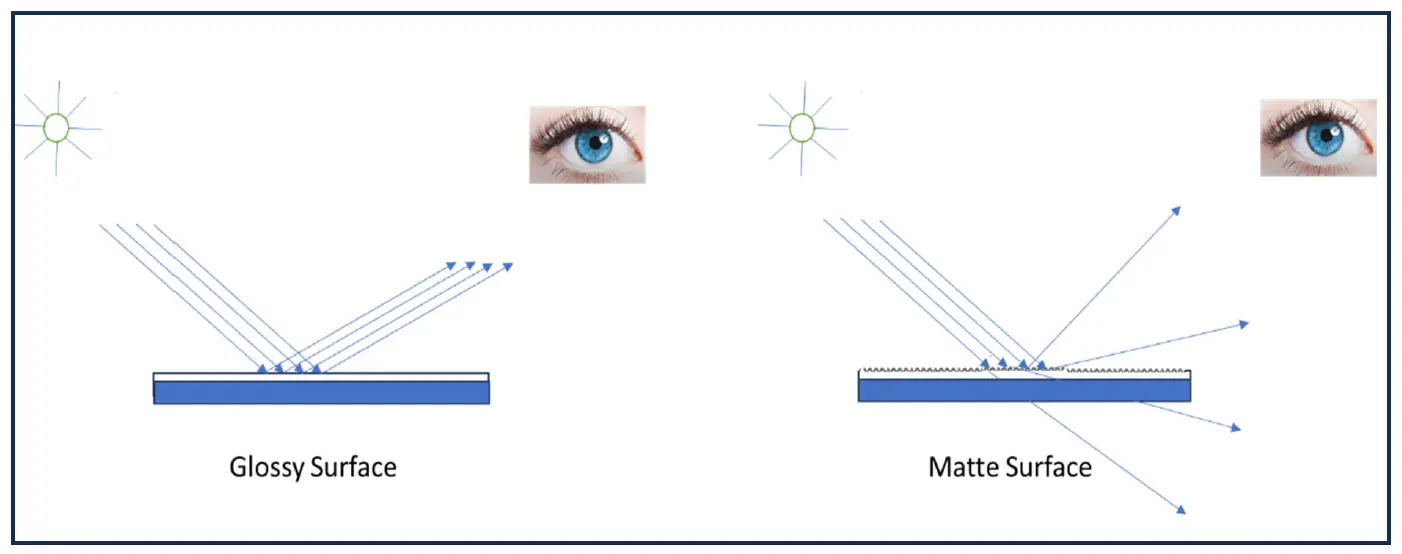
The high gloss of a painted surface is achieved by a smooth surface, which results in specular reflection of light. In contrast, to achieve a matte surface, it is required to have microscale roughness on the coating surface that scatters light in a diffused manner, enabling a low gloss appearance. In other terms, “gloss” stands for a complete reflection of light from the view that the surface scatters and reflects incident light at a wide-angle. The greater the scattering angle, the lower the observed gloss, giving surface a matte appearance. The figure below (Figure 1) represents the difference between reflection of light from a glossy and matte surface. The gloss level of coating depends on the particle size and PVC of the matting agent used [16]. Particle size, particle size distribution, and compatibility play a crucial role in achieving matte finishes. Larger particles generally increase surface roughness and enhance light scattering, resulting in a stronger matting effect; however, excessively large particles can compromise film integrity and lead to surface defects. A narrow particle size distribution is essential for achieving uniform scattering and a consistent matte finish. Furthermore, the refractive index of the matting agent should differ sufficiently from that of the binder to maximize scattering efficiency. Equally important is chemical compatibility, which prevents agglomeration, sedimentation, and poor dispersion during formulation, ensuring stable performance and high-quality matte finish [17].
In a typical Gloss-meter, the commonly used incident light angles are 20°, 60°, and 85°. The choice of measurement angles is application specific, e.g., Automotive coating typically requires a 20° angle, while wood coating usually requires 60° angle. A coating with gloss value greater than 70 gloss units is considered a high-gloss; gloss units between 30 and 70 indicate a semi-gloss coating; and gloss units below 30 are considered matte coatings.
3. Conventional Matting Agents
A few conventional matting agents are already being used to have the matting effect in coating. They work primarily by creating micro-roughness on the coating surface, that scatters incident light and reduces specular reflection. Conventional matting agents are generally used in solventborne, waterborne, and UV-curable coating for wood, furniture, automotive, and industrial applications. These include silica, silica gel, waxes, fillers, and nanomaterials. Details of each conventional matting agent are described below.
3.1. Silica
Silica (mainly precipitated and pyrogenic) is highly effective in reducing the gloss for both waterborne and solventborne systems. Due to the porous nature of silica, it provides a matting effect even at lower concentrations. The main advantages of using silica are its high wet film clarity, economical availability, and efficiency at lower concentrations. It also provides scratch-resistant properties to the dried paint film. The disadvantage is that, due to its very lightweight nature, it is difficult to incorporate into the coating formulation [18]. Commercially available matte silicas in this category are Acematt TS 100, Acematt OK 412, Acematt OK 500, and Acematt OK 520, etc.
3.2. Silica Gel
Silica gel is the amorphous form of silica composed of nearly 100% silicon dioxide produced synthetically in a liquid process. However, it exhibits significant differences in physical properties compared to other specialty silica. Due to its large pore size and high surface area, it shows unique properties, including resistance to shear stresses during paint manufacturing and application. In addition, unlike conventional silica, it provides desirable rheological properties to wet paints [19], e.g., Syloid ED 30.
3.3. Waxes
Waxes are mainly added to improve the surface properties of any coatings by modifying surface free energy. Other than that, it also gives a matting effect when incorporated into coatings in various forms, such as micronized, micro powders, emulsified, and dispersions. Depending on the type of wax used and the particle size of the wax, it acts as a matting agent. Waxes are less efficient than conventional silica, but in deep matte coatings, the quantity of silica is very high, therefore, it creates a strong increase in viscosity and enhanced thixotropy. Therefore, it’s better to use wax in that case due to its ability to float on the surface [19], e.g., Polyethylene wax, polypropylene wax, etc. Polyethylene wax-based additives, such as CeridustTM 3715 from Clariant and NduroMatt® from Honeywell (Charlotte, NC, USA), are commonly used in wood coating applications. They provide excellent matte effect, improved slip properties, a pleasant tactile feel, and good clarity for high-quality wood finishes.
3.4. Fillers
Fillers are also used to reduce the gloss. After drying the paint film, filler particles create micro roughness in the surface of the paint film that helps to reduce the reflection of light, thus giving a matte finish film. The disadvantage of using filler is that it has a recommended level. When used above its recommended level, it decreases the flatness and mechanical strength of the film, e.g., talc, calcium carbonate, etc.
3.5. Nanomaterials
Nanomaterials are also used as a matting agent. These are especially used for high solid coatings. For low solid coatings, maintaining the viscosity after adding a matting agent is a big challenge. Therefore, to maintain that viscosity of coatings, sometimes nanomaterials are dispersed in the monomer, e.g., nanogas-phase SiO2 powder, montmorillonite nanosheets, Nano carbon, etc. [20,21].
Comparative details of conventional matting agents are summarized in Table 1.
Table 1. Comparison of Conventional Matting Agents: Composition, Mechanism, Applications, and Performance.
|
Matting Agent |
Composition |
Mechanism |
Applications |
Advantages |
Limitations |
Examples |
|---|---|---|---|---|---|---|
|
Silica |
Precipitated/Pyrogenic silica |
Creates micro-roughness; porous structure |
Waterborne & solventborne coatings |
High wet film clarity, economical, efficient at low concentration, and scratch resistance |
Difficult to disperse (lightweight nature) |
Acematt TS 100, OK 412, OK 500, OK 520 |
|
Silica Gel |
Amorphous silica (synthetic) |
Large pore size & surface area |
Waterborne & solventborne coatings |
Shear-resistant during manufacturing, improves rheology |
Higher cost than standard silica |
Syloid ED 30 |
|
Waxes |
Polyethylene, polypropylene waxes |
Alters surface free energy; floats on the surface |
Wood coatings, furniture finishes |
Excellent matting effect in deep matte, improved slip, tactile feel, and clarity |
Less efficient than silica; limited matting effect alone |
CeridustTM 3715, Honeywell NduroMatt® |
|
Fillers |
Talc, calcium carbonate |
Creates micro-roughness after drying |
Industrial coatings, general-purpose paints |
Economical, easy to use |
Excess use reduces flatness & mechanical strength |
Talc, CaCO3 |
|
Nanomaterials |
Nano SiO2, montmorillonite, nano-carbon |
Dispersed in monomer for viscosity control |
High-solid & UV-curable coatings |
Maintains viscosity, enables anti-glare properties |
Costly, it requires dispersion technology |
nanogas-phase SiO2, Nano carbon |
4. Organically Modified Matting Agents
Herein, organic modification refers to existing inorganic matting agents that have been functionalized with organic molecules, such as coupling agents through process optimization or binder modification to get the desired matting effect. Two types of modifications of matting agents are reported, including a modified silica matting agent and a self-matting acrylic or polyurethane dispersion.
4.1. Modified Silica
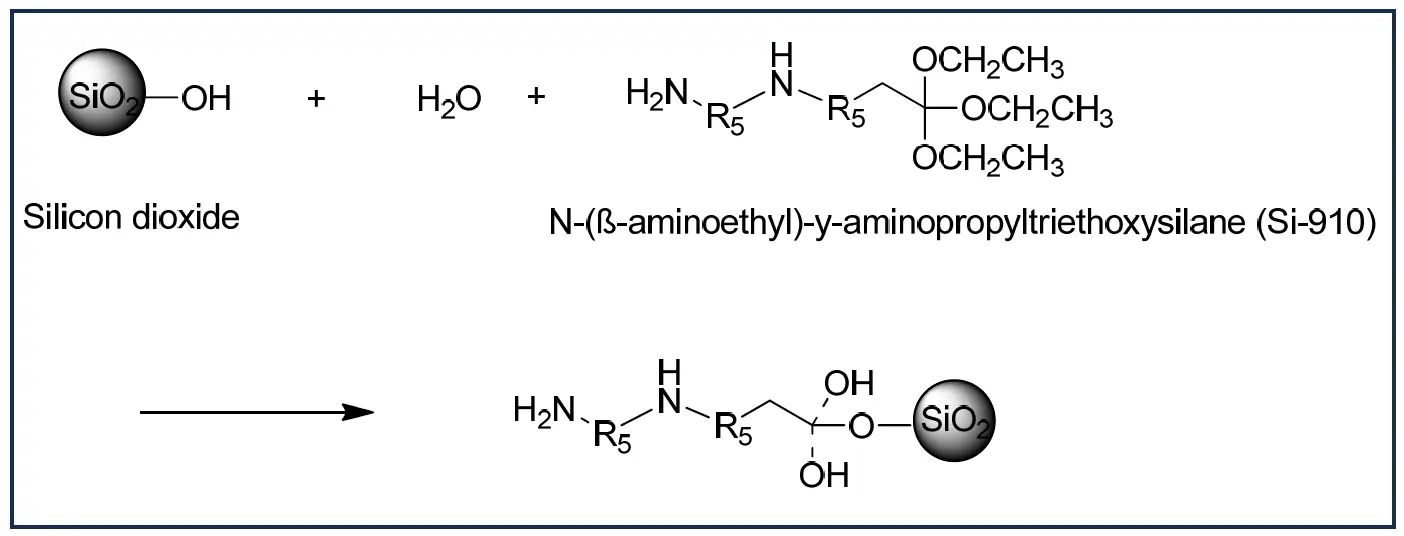
Researchers have devoted efforts to modifying silica surfaces to overcome problems associated with the silica-based matting agents. Various modification methods have been reported in the literature. Hui et al. synthesized modified silica by employing N-(β-aminoethyl)-γ-aminopropyltriethoxysilane in an aqueous solution to functionalize SiO2 (Scheme 1), which was subsequently incorporated directly into the waterborne polyurethane (WBPU) prepolymer for emulsification. Fourier transform infrared (FTIR) spectroscopy revealed that this mechanism promotes chain extension, allowing the modified silica surface to acquire active amino groups for the isocyanate reaction. This technology generated a novel hydrophilic and environmentally friendly WBPU matte coating with modified silica branched into polyurethane chains, claiming that the gloss is <5 at 60°. The physical properties of the silica modified matte WBPU resin ware reported are the mean particle size (D50) was approximately 6.1 μm, viscosity at 25 °C was 350 mPa·s, solids were between 39.5–40.5% and the VOC reported is <0.1% [22]. The reported physical properties of the silica-modified matte WPU resin include a mean particle size (D50) of approximately 6.1 μm, a viscosity of 350 mPa·s at 25 °C, solids content between 39.5–40.5%, and a VOC level of less than 0.1%.
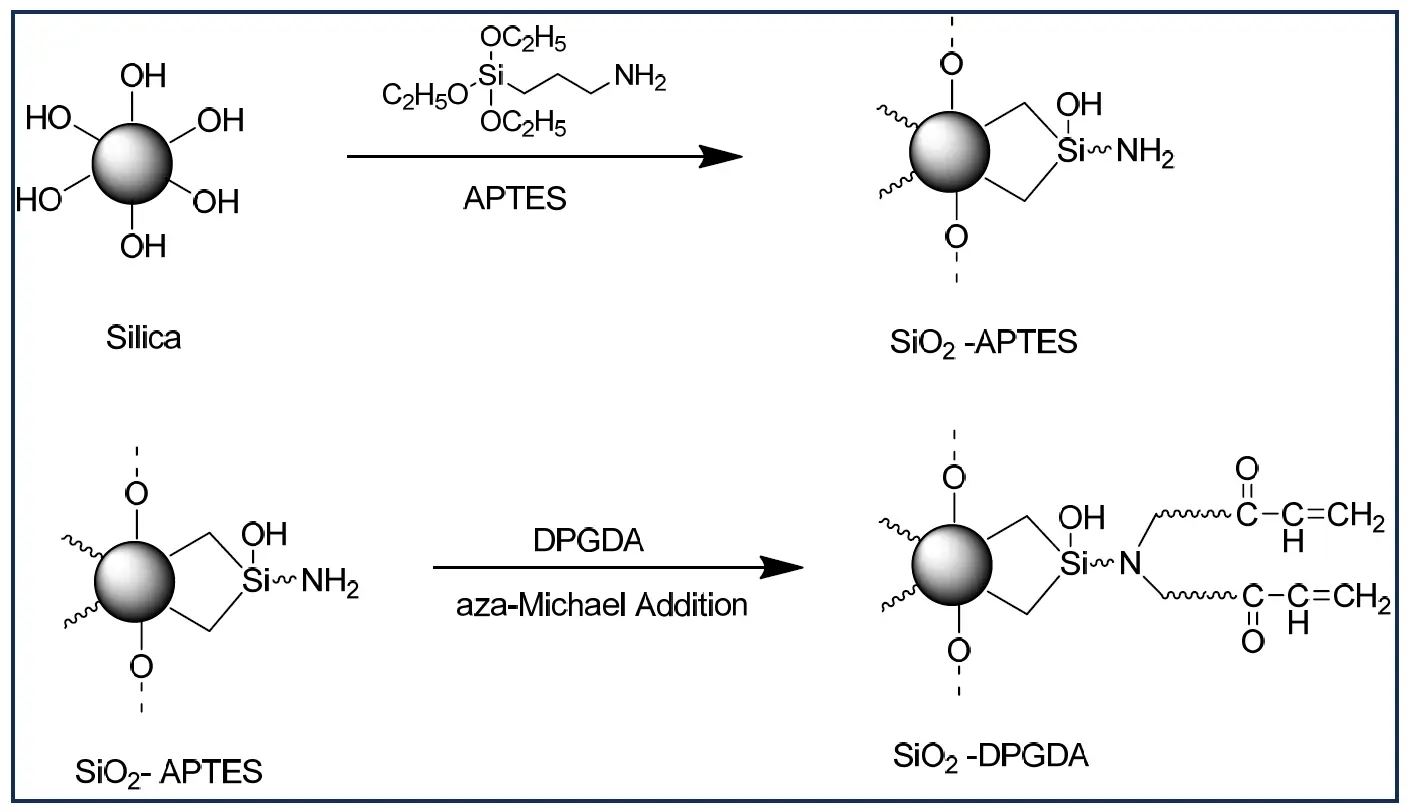
Calvez et al. reported that the micro-sized silica gives matte surfaces with superior abrasion resistance. The silica surface was transformed using a two-step synthesis process: In the first step, the silica surface was modified using (3-aminopropyl) triethoxysilane (APTES) via a silanization process, and then the amino silane was further reacted with a di-propylene glycol diacrylate (DPGDA) via aza-Michael mechanism (Scheme 2). By using amine functionality on the silica surface, a wide range of monomers and oligomers are grafted on to silica. As a result, the performance of silica in the coating was modulated by using chain extension, reactivity, compatibility with UV-curable paint formulations, and matting efficiency. The efficiency of grafting was determined by elemental analysis, 29Si and 13C CP MAS NMR, and thermogravimetric analysis. The extent of conversion and polymerization kinetics of UV-curable coatings was determined by using FTIR and DSC with UV lamps, respectively. The particle size (D50) of DPGDA-modified silica reported is 12.9 μm, and the polydispersity index (PDI) ranges from 0 to 1. The larger particle size of the modified silica created micro-roughness on the coating surface, as confirmed by 3D profilometry. The modified silica was tested in a UV-curable coating formulation for matting efficiency in comparison with the commercial matting agent Deurex S3009M from DEUREX AG, Elsteraue, Germany. Loadings of 3–10% of the modified silica showed better matting efficiency than Deurex S3009M. The gloss measured at a 60° angle was 13.6 at 10% loading of modified silica, compared to 49.6 at 10% loading of Deurex S3009M [23].
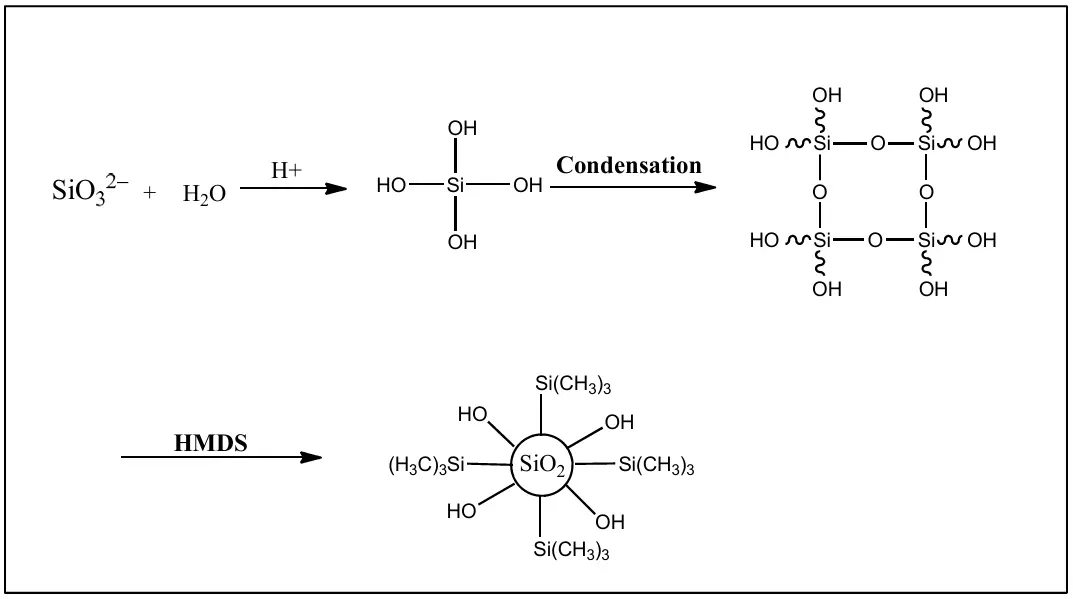
Xu et al. reported the preparation of hexamethyldisilazane (HMDS)-modified silica (referred to as H-SiO2) by a liquid-phase in-situ surface modification process using HMDS as the surface-modifying reagent (Scheme 3). To the solution of sodium metasilicate in deionized water, hydrochloric acid was added under stirring at an elevated temperature. Subsequently, a solution of HMDS in ethanol was added dropwise to obtain a suspension of H-SiO2. Next, the author prepared paint using composite acrylic amino (AA) and H-SiO2 paints and coatings by mixing H-SiO2 with AA baking paint. Further characterization was done to confirm the structure, viscosity, gloss, and transmittance of AA/H-SiO2 composite paints in relation to the particle size of H-SiO2. The gloss achieved by using H-SiO2 is up to 10 units, which is better than commercial silica matting agent OK 520. The hardness and adhesion of the AA paint were optimized using modified silica. AA paint incorporating H-SiO2 demonstrated superior performance compared to OK 520, exhibiting lower viscosity and lower gloss in matte coatings [24].
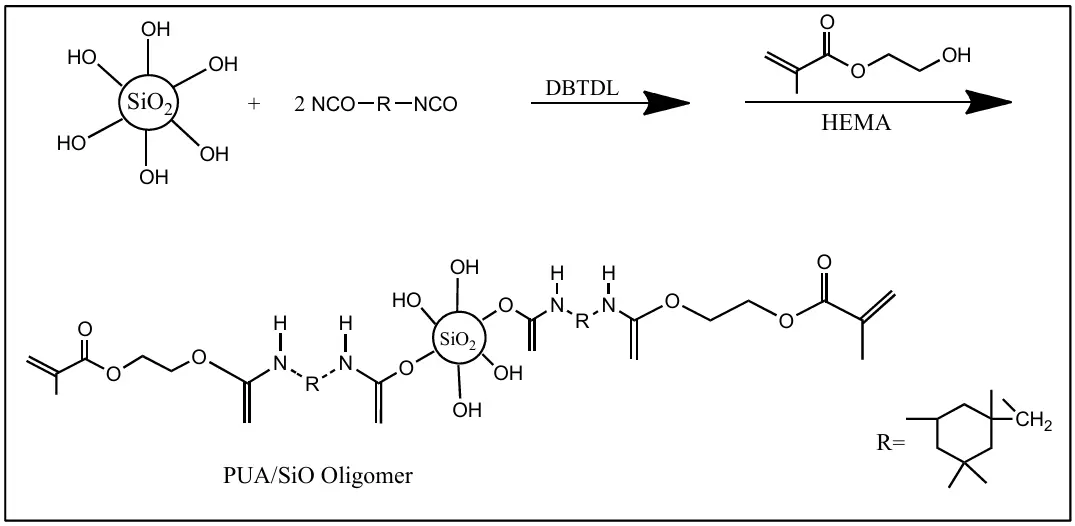
Ziyuan Yang et al. demonstrated an in-situ polymerization process for evenly distributing silica matting agents with varying particle sizes in a polyurethane (PU) acrylate solution. PUA/SiO2 matting composites are created through in-situ polymerization. Isophorone diisocyanide (IPDI) was reacted with hydroxy groups in polypropylene glycol and silica using Dibutyltin dilaurate (DBTDL) as a catalyst. 2-hydroxyethyl methacrylate (HEMA) was used as an end sealer to create a PU acrylate/SiO2 oligomer with a carbon-carbon double-bond end group (Scheme 4). This silica matting agent was evaluated in UV curable matte coating composition. FTIR was used to confirm the covalent bonding of silica with the polymer chain, and SEM was used to determine the particle size of modified silica. The study examined how silica particle sizes affect thermal stability, surface gloss, and wear resistance in composite materials in UV curable coatings. When the silica particle size was 2.5 µm, the gloss of the composite was observed to be 14.1 gloss units [25].
Juhua Ou et al. reported an acrylic resin/SiO2/polymethyl urea resins (PMU) composition with various amounts of SiO2 and PMU using a physical mixing approach. Initially, a modified silica dispersion was prepared by reacting TEOS and triethoxyvinylsilane (VTEO) in ethanol and water. Subsequently, hybrid material was synthesized by producing an acrylic emulsion, followed by the addition of modified silica and PUM (Scheme 5). The structure, transmittance, thermal, and optical properties of the material were analyzed using FTIR, TEM, SEM, UV-Vis, TGA, and gloss meter. Using 7% by weight of modified silica, the gloss achieved with this approach is as low as 18.9 gloss units. This research is dedicated to study the impact of composite structures on hybrid membranes, aiming to achieve a matting effect on the coating surface [7].
Moravek et al. treated precipitated silica with 3-triethoxysilylpropylamine using silica with particle sizes ranging between 10–22.5 micron. Then, formulate a clearcoat using amine-functional silica and apply it to the steel panels. The gloss of the dried clearcoat film is reported in the range of 5.3–13.4 gloss units at 60° [26].
The details of modified silica approaches are summarized in Table 2.
Table 2. Comparison of silica surface modification approaches for matting agents.
| Silica Modification Method | Particle Size (D50) | Gloss at 60° | Application | Key Advantages | Refs. |
|---|---|---|---|---|---|
| Amino-silane modified silica in WBPU | ~6.1 μm | <5 | Waterborne PU matte coatings | Ultra-low gloss, eco-friendly (VOC < 0.1%), strong chain integration | [14] |
| Acrylate-modified silica via aza-Michael reaction | 12.9 μm | 13.6 | UV-curable coatings | Superior scratch resistance, better than commercial matting agents | [15] |
| HMDS-modified silica in AA baking paint | Not specified | ~10 | Baking paint system | Lower viscosity, better adhesion, improved matte effect vs. commercial OK 520 | [16] |
| PUA/SiO2 via in-situ polymerization | 2.5 μm | 14.1 | UV-curable matte coatings | Enhanced thermal stability and wear resistance | [17] |
| Acrylic resin/SiO2/PMU hybrid (physical mixing) | Not specified | 18.9 | Hybrid membranes | Simple process, moderate matting effect | [7] |
| Amine-functional silica in clearcoat | 10–22.5 μm | 5.3–13.4 | Clearcoat applications | Good matte finish, suitable for automotive or industrial clearcoats | [18] |
4.2. Self-Matting Polymer
The self-matting mechanism in modern coatings relies on creating micro-roughness on the surface during drying, which scatters incident light and reduces specular reflection, eliminating the need for external matting agents. The recent trend in self-matting polymers encompasses two primary pathways: WBPU and waterborne acrylate (WBA) polymers. Many literatures focus mainly on WBPU, while a few focus on aqueous acrylate. Below are the details of self-matting polymers reported in the literature, including synthesis, matting behavior, and applications.
4.2.1. Waterborne Polyurethane Self-Matting Polymer
In WBPU systems, hydrophilic chain extenders such as carboxylic and sulfonic acids promote the formation of wrinkled spherical latex particles. As water evaporates, these particles migrate and partially embed on the surface, generating a rough texture. This micro-roughness, with optimal particle sizes, achieves gloss levels as low as possible. Qiwen Yong et al. prepared a self-matting paint of WBPU by combining self-emulsification and prepolymer methods. In this study, Poly(tetramethylene oxide glycol) (PTMG) and dimethylol propionic acid (DMPA) based polyurethanes were prepared at 65 °C for 3 h using an organic bismuth catalyst (KRBi-28) and IPDI. The conventional dibutyl amine-based titration method was utilized to determine the isocyanate content during the reaction. At 50 °C, 100% neutralization was achieved by feeding triethyl amine into the reactor for 30 min. Following a vigorous stirring period, the flask was filled with 2-[(2-aminoethyl)amino] ethanesulphonate sodium (AAS) salt, which had been dissolved in 80% w/w deionized water. The inclusion of EDA in DI water for 30 min was the last step in the chain extension process. WBPU emulsions have a solid component of roughly 30%. After the processing of the coating, it was applied at different thicknesses and then observed that the thickness between 2.5–3.0 microns show equilibrium between particle size distribution and microroughness to obtain perfect matte films. The self-matting polymer sample with roughness parameters Ra and Rq (1.04 and 1.24, respectively) achieved the lowest gloss value of 10 gloss units at 60°. This WBPU gives excellent hardness, adhesion, and stability to the coatings [27].
Qiwen Yong et al. reported a castor oil modified matte crosslinked WBPU by using castor oil and bisphenol A type epoxy resin based crosslinker. Authers observed a decrease in coating gloss with increasing in castor oil levels. The resulting coating films exhibited good adhesion, and a tender touch feel [28]. In another study, authors also reported inherently matte composite based on combination of crosslinked WBPU and silicon modified WBPU. The incorporation of silane coupling agents improved the overall properties of the composite. The composite was able to create micro-surface roughness, while the film formation process enabled a lower gloss of the composite. The authors noted that the 50:50 composite resins showed better performance over crosslinked WBPU alone. The coatings exhibit a matte effect with good transparency, elongation, hardness, and storage stability [29].
Qiwen Yong et al. reported the synthesis of solvent-free WBPU using a combination of sulfonic and carboxylic hydrophilic chain-extending agents through a two-step pre-polymerization and self-emulsification process. The self-matting effect is attributed to the formation of numerous spherical particles (0.8–3.0 μm) on the film surface during the drying process. Among the tested formulations, one of the samples achieved the lowest gloss achieved is 6 gloss units at 60°, delivering an excellent matte finish [30].
Gao et al. made a matte WBPU coating using polyurea hollow microspheres. The microspheres were prepared by suspension polymerization using IPDI, 2,4-diaminobenzenesulfonic acid sodium salt, and polyvinylpyrrolidone (PVP) in water/acrylonitrile solution. PVP helped to give surface porosity and roughness to the polyurea particles. When 5% polyurea hollow microspheres were added to the WBPU coating, the gloss of the coating decreased by approximately 80% [31].
4.2.2. Waterborne Acrylate Self-Matting Polymer
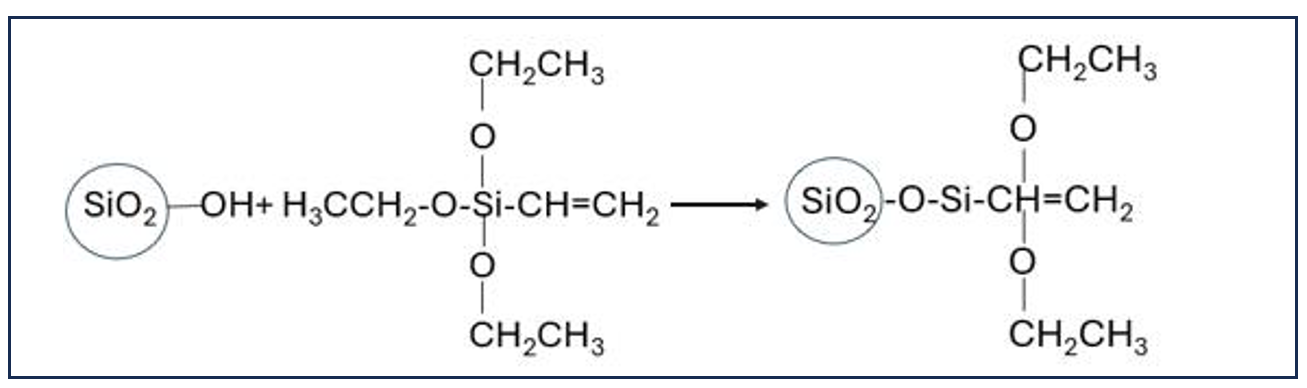
Taoling Xie et al. reported the synthesis of a self-matting acrylic resin by using hydrolysable silane and ϒ-methacryloxypropyl trimethoxyl silane (ϒ-MPS). The ϒ-MPS is polymerized with other acrylic monomers via core-shell polymerization. The alkoxy groups of silicon are hydrolyzed with water, yielding a silanol functional resin. These silanol functionalities further condense to a crosslinked structure like silica (Figure 2). The lower gloss of the coating surface is achieved through a combination of core-shell structures, the presence of silicon moieties, and the incompatibility of acrylic and silica spheres, resulting in micro-phase separation and a micro-surface rough morphology. The addition of ϒ-MPS to the polymerization process helped improve the mechanical properties and water resistance of the resulting film. The gloss value of the coated film was reported to be 6.3 gloss units at a 60° [32].
Poly(methyl methacrylate) (PMMA) microspheres were prepared by Wang et al. via one-step dispersion polymerization, using methyl methacrylate (MMA) as the monomer, polyvinylpyrrolidone (PVP) as the stabilizer, and sodium styrene sulfonate (NaSS) as a functional co-monomer. The Authors studied the effect of PVP and NaSS concentrations on the particle size and dispersity of microspheres. They concluded that the matting effect is attributed to the size of the particles, rather than the distribution of microspheres. The size of the PMMA microspheres is inversely proportional to NaSS or PVP. They coated the bulk mixture of WBPU with PMMA microspheres onto a leather substrate to achieve surface roughness, as the PMMA microspheres are compiled on a dried coating surface. Lower gloss values (approximately 20 gloss units) are observed when the microsphere particle size is larger (1.51 µm). The authors illustrated the relationship between gloss and surface roughness of WPU coatings containing PMMA microspheres [33].
Fluorinated waterborne resins were synthesized by Lopez et al. by mini-emulsion polymerization using 1H,1H,2H,2H-perfluorodecyl acrylate (PFDA), 2-ethylhexyl acrylate (2EHA), and methacrylic acid (MAA) as monomers. The process employed high-pressure homogenization to achieve stable mini emulsions with droplet sizes around 150 nm, followed by polymerization at 70 °C to full conversion. These resins were evaluated in commercial matte paint formulations. Matting efficiency improved with increasing fluoropolymer content, with copolymer C80 exhibiting the strongest matte effect. When combined with one-third of the original matting agent, gloss values dropped below the commercial matte reference, achieving the lowest gloss of 38 gloss units at 20° and 63 gloss units at 60°. The coating with fluro-acrylates shows hydrophobicity in comparison to regular acrylic binders [34].
5. Challenges and Future Perspectives
Despite significant progress in organically modified matting agents and self-matting polymers, several challenges remain that limit their extensive acceptance. Surface functionalization of silica using silane or acrylate groups, and the synthesis of self-matting polymers, involve multi-step reactions and stringent process control. This can lead to an increase in manufacturing costs compared to conventional silica-based matting agents, limiting commercial scalability. Achieving universal compatibility with both waterborne and solvent-based coatings, while maintaining low gloss and mechanical integrity, is also another challenge.
The future of matte coating technology is expected to advance through the development of organic-inorganic hybrid systems that synergistically combine the benefits of modified silica and self-matting polymers, ensuring broad compatibility and consistent performance. Emerging research on smart, stimuli-responsive polymers that can dynamically modulate surface roughness and gloss under varying environmental conditions presents promising opportunities for adaptive coatings. Furthermore, the integration of predictive modelling and advanced surface characterization techniques, such as SEM and AFM, will enable precise evaluation of matting efficiency and optimization of particle-polymer interactions. Finally, simplifying synthesis pathways and adopting continuous manufacturing technologies will be critical to lowering production costs and facilitating large-scale industrial implementation of next generation matting agents.
6. Conclusions
This article provides a comprehensive overview of the evolution and advancements in matting agents for coating applications, highlighting the limitations of conventional agents and the promising potential of organically modified alternatives. Traditional matting agents such as silica, waxes, fillers, and nanomaterials, though effective to some extent, often suffer from drawbacks including poor compatibility with binder systems, sedimentation, and compromised mechanical properties. In contrast, polymeric matting agents, including organically modified silica and self-matting polymers such as WBPU and waterborne acrylates, offer superior compatibility, uniform dispersion, and improved film integrity. Organically modified matting agents, particularly those involving silane and acrylate functionalization of silica, have demonstrated significant improvements in gloss reduction, compatibility, and mechanical performance. These modifications enable the creation of micro-rough surfaces that effectively scatter light, achieving gloss levels below 10 gloss units and enhancing the aesthetic and functional qualities of coatings. Furthermore, the development of self-matting polymers, especially waterborne polyurethane and waterborne acrylate systems, has opened new pathways for achieving uniform matte finishes without the need for any traditional matting agents. These polymeric systems inherently generate micro-roughness during film formation, eliminating the need for additional matting additives. These polymers offer advantages such as improved film formation, enhanced mechanical properties, and better outdoor performance.
There is substantial scope for innovation in designing universal organic and or polymeric matting agents. The integration of renewable and bio-based chemistries into matting agent development aligns with sustainability goals and provides exciting opportunities to researchers.
Acknowledgments
We extend our heartfelt thanks to the management of “Asian Paints Limited” for their support in completing the work. We are thankful to Rajeev Kumar Goel for his constant support in publishing this review article.
Author Contributions
Conceptualization, R.M.P.; Methodology, R.M.P.; Software, U.G.; Validation, R.M.P.; Formal Analysis, U.G.; Investigation, R.M.P.; Writing—Original Draft Preparation, (U.G.; Writing—Review & Editing, R.M.P.; Visualization, U.G.; Supervision, R.M.P.; Project Administration, R.M.P.
Ethics Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created in this study.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xie T, Kao W, Sun L, Wang J, Dai G, Li Z. Preparation and characterization of self-matting waterborne polymer-An overview. Prog. Org. Coat. 2020, 142, 105569. DOI:10.1016/j.porgcoat.2020.105569 [Google Scholar]
- Li G, Tan Y, Li Z, Zhou G, Yu X, Nie Q, et al. Advances in waterborne polyurethane matting resins: A review. Appl. Surf. Sci. Adv. 2024, 19, 100557. DOI:10.1016/j.apsadv.2023.100557 [Google Scholar]
- Maskery SE. Development and applications for matting agents. Pigment. Resin Technol. 1973, 2, 11–19. DOI:10.1108/eb040916 [Google Scholar]
- Gunde MK, Kunaver M, Cekada M. Surface analysis of matt powder coatings. Dye. Pigment. 2007, 74, 202–207. DOI:10.1016/j.dyepig.2006.01.049 [Google Scholar]
- Nsib F, Ayed N, Chevalier Y. Matting Agent Concentration and its Effect on the color and the rheology of matted coatings. J. Appl. Sci. 2008, 8, 1527–1533. DOI:10.3923/jas.2008.1527.1533 [Google Scholar]
- Schubert J, Kuhlmann R, Christian D. Efficient Matting Agents Based on Precipitated Silicas. US8012253B2, 6 September 2011. [Google Scholar]
- Ou J, Zhang M, Liu H, Zhang L, Pang H. Matting films prepared from waterborne acrylic/micro-SiO2 blends. J. Appl. Polym. Sci. 2014, 132, 41707. DOI:10.1002/app.41707 [Google Scholar]
- Nano-Scale Silica Matting Agent Market Report: Trends, Forecast and Competitive Analysis to 2030. Lucintel, 27 July 2025. Available online: https://www.lucintel.com/nano-scale-silica-matting-agent-market.aspx (accessed on 12 December 2025).
- Alexander K, Matthias M, Steve B, Gu F. Next Generation Matting Agents for Highly Durable Waterborne Coatings. Coatings World, ISSN 1527-1129, accessed on 1 February 2020. Available online: https://www.coatingsworld.com/next-generation-matting-agents-for-highly-durable-waterborne-coatings/ (accessed on 12 December 2025).
- Cao X, Ge X, Chen H, Li W. Effects of trimethylol propane and AAS salt on properties of waterborne polyurethane with low gloss. Prog. Org. Coat. 2017, 107, 5–13. DOI:10.1016/j.porgcoat.2017.02.021. [Google Scholar]
- Matthew TL. Novel matting agents for low VOC coatings. In Proceedings of the 40th Annual International Waterborne, High-Solids, and Powder Coatings Symposium, New Orleans, LA, USA, 4–8 February 2013. [Google Scholar]
- Brambila C, Boyd P, Keegan A, Sharma P, Vetter C, Ponnusamy E, et al. A comparison of environmental impact of various silicas using a green chemistry evaluator. ACS Sustain. Chem. Eng. 2022, 10, 5288–5298. DOI:10.1021/acssuschemeng.2c00519 [Google Scholar]
- Jimenez Lopez AM, Hincapie-Llanos GA. Identification of factors affecting the reduction of VOC emissions in the paint industry: Systematic literature review-SLR. Prog. Org. Coat. 2022, 170, 106945. DOI:10.1016/j.porgcoat.2022.106945 [Google Scholar]
- Clingerman D. Silane-Modified Silicas as Functional Matting Agents. CoatingsTech 2021, 18, 32–36. Available online: https://www.paint.org/coatingstech-magazine/articles/silane-modified-silicas-as-functional-matting-agents/ (accessed on 12 December 2025).
- Yong Q, Xu D, Liu Q, Xiao Y, Wei D. Advances in polymer-based matte coatings: A review. Polym. Adv. Technol. 2022, 33, 5–19. DOI:10.1002/pat.5508 [Google Scholar]
- Lamy R, Zunic E, Steding R, Aamodt A. Preparation of stable slurries of spherically shaped silica for coatings. Prog. Org. Coat. 2011, 72, 96–101. DOI:10.1016/j.porgcoat.2011.03.020 [Google Scholar]
- Sylvia H, Christopher H. Matting energy-curable coatings through novel dispersant technology. In Proceedings of the Rad Tech UV&EB Technology Expo & Conference, Chicago, IL, USA, 16–18 May 2018. [Google Scholar]
- Gu F, Pryor JN. Silica-Based Matting Agents and Methods of Making and Using the Same. WO2019028312A1, 7 February 2019. [Google Scholar]
- Kretzschmar M, Lehnert HR. Matting Agents. EP2099873B1, 4 November 2015. [Google Scholar]
- Hu L, Yang Z, Zhang X. Fabrication and evaluation of dual function PMMA/nano-carbon composite particles for UV curable anti-glare coating. Prog. Org. Coat. 2016, 101, 81–89. DOI:10.1016/j.porgcoat.2016.07.020 [Google Scholar]
- Ding Z, Li J, Xin W, Zhang G, Luo Y. Low gloss waterborne polyurethane coatings with anti-dripping and flame retardancy via montmo rillonite nanosheets. Prog. Org. Coat. 2019, 136, 105273. DOI:10.1016/j.porgcoat.2019.105273 [Google Scholar]
- Ma H, Liu Y, Guo J, Chai T, Suming J, Zhou Y, et al. Synthesis of a novel silica modified environmentally friendly waterborne polyurethane matting coating. Prog. Org. Coat. 2020, 139, 105441. DOI:10.1016/j.porgcoat.2019.105441 [Google Scholar]
- Calvez I, Szczepanski CR, Landry V. Preparation and characterization of low gloss UV-curable coatings based on silica surface modification using an acrylate monomer. Prog. Org. Coat. 2021, 158, 106369. DOI:10.1016/j.porgcoat.2021.106369 [Google Scholar]
- Xu Q, Ji T, Tian Q, Su Y. Structural Adjustment of In-Situ Surface-Modified Silica Matting Agent and Its Effect on Coating Performance. Nano 2018, 13, 1850137. DOI:10.1142/S1793292018501370 [Google Scholar]
- Yang Z, Wu J, Ma G, Hou C, Niu Y, Duan H, et al. Effect of the particle sizes of silica on the properties of UV-curing matting coatings. J. Coat. Technol. Res. 2021, 18, 183–192. DOI:10.1007/s11998-020-00395-4 [Google Scholar]
- Moravek SJ, Mohnot S, Thomas SJ. Methods of Improving Burnish Resistance Using Curable Film-Forming Compositions Demonstrating Burnish Resistance and Low Gloss. US20150099129A1, 15 May 2018. [Google Scholar]
- Yong Q, Nian F, Liao B, Guo Y, Huang L, Wang L, et al. Synthesis and surface analysis of self-matt coating based on waterborne polyurethane resin and study on the matt mechanism. Polym. Bull. 2016, 74, 1061–1076. DOI:10.1007/s00289-016-1763-7 [Google Scholar]
- Yong Q, Liao B, Huang J, Guo Y, Liang C, Pang H. Preparation and characterization of a novel low gloss waterborne polyurethane resin. Surf. Coat. Technol. 2018, 341, 78–85. DOI:10.1016/j.surfcoat.2018.01.012 [Google Scholar]
- Yong Q, Liao B, Ying G, Caizhen L. Structure and surface properties of a novel bulk-matte waterborne polyurethane coating composite. J. Coat. Technol. Res. 2018, 15, 993–1002. DOI:10.1007/s11998-017-0030-7 [Google Scholar]
- Yong Q, Nian F, Liao B, Huang L, Wang L, Pang H. Synthesis and characterization of solvent-free waterborne polyurethane dispersion with both sulfonic and carboxylic hydrophilic chain-extending agents for matt coating applications. RSC Adv. 2015, 5, 107413–107420. DOI:10.1039/C5RA21471H [Google Scholar]
- Gao Q, Jia L, Zhang A, Wang L, Peng H, Wang W, et al. Synthesis of porous polyurea microspheres for matting coating. J. Mater. Sci. 2022, 57, 19730–19742. DOI:10.1007/s10853-022-07893-3 [Google Scholar]
- Xie T, Kao W, Zhang Z, Liu Y, Li Z. Synthesis and characterization of organosilicon modified self-matting acrylate polymer: Insight into surface roughness and microphase separation behaviour. Prog. Org. Coat. 2021, 157, 106300. DOI:10.1016/j.porgcoat.2021.106300 [Google Scholar]
- Wang T, Sun Z, Liang F, Fan H, Xiang J, Chen Y. Polymethyl methacrylate microspheres with matting characteristic prepared by dispersion polymerization. Int. J. Polym. Anal. Charact. 2019, 24, 731–740. DOI:10.1080/1023666X.2019.1670393 [Google Scholar]
- Lopez AB, Bohórquez SJ, Meeuwisse M, Mestach D, de la Cal JC, Asua JM. Self-matting waterborne fluoropolymers. Prog. Org. Coat. 2018, 116, 57–69. DOI:10.1016/j.porgcoat.2017.12.003 [Google Scholar]