Breath and Life: Emerging Nanotechnologies for Cystic Fibrosis Therapy
Received: 26 November 2025 Revised: 12 December 2025 Accepted: 25 December 2025 Published: 29 December 2025
© 2025 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Cystic fibrosis (CF) remains one of the most prevalent and fatal monogenic disorders worldwide, stemming from pathogenic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which is inherited from asymptomatic carrier parents [1,2]. Based on the available data, it is estimated that there are more than 180,000 people with CF worldwide [1]. The absence or malfunction of the CFTR protein causes the accumulation of thick mucus, which blocks the airways and leads to repeated infections and lung damage. Although CF manifests as a systemic multi-organ disease affecting the pancreas and other systems, progressive obstructive lung disease remains the primary cause of morbidity and premature mortality [3].
Traditional therapies primarily focused on treating symptoms [4], including mucolytics (e.g., dornase alfa) to enhance mucus clearance and antibiotics to manage chronic infections. Breakthroughs in the development of therapies that target the underlying cause of CF are made possible by the advent of CF modulators, such as Trikaftor [5]. However, a subset of patients, such as those with nonsense mutations, does not respond to these drugs. Furthermore, since Trikaftor is administered systemically, its delivery to the respiratory epithelium is often limited by biological barriers. Consequently, enhancing bioavailability to ensure sufficient therapeutic concentrations in the respiratory tract without inducing systemic toxicity remains a critical objective. These unresolved challenges collectively underscore the urgent need for advanced drug delivery systems characterized by precise targeting and high therapeutic efficiency.
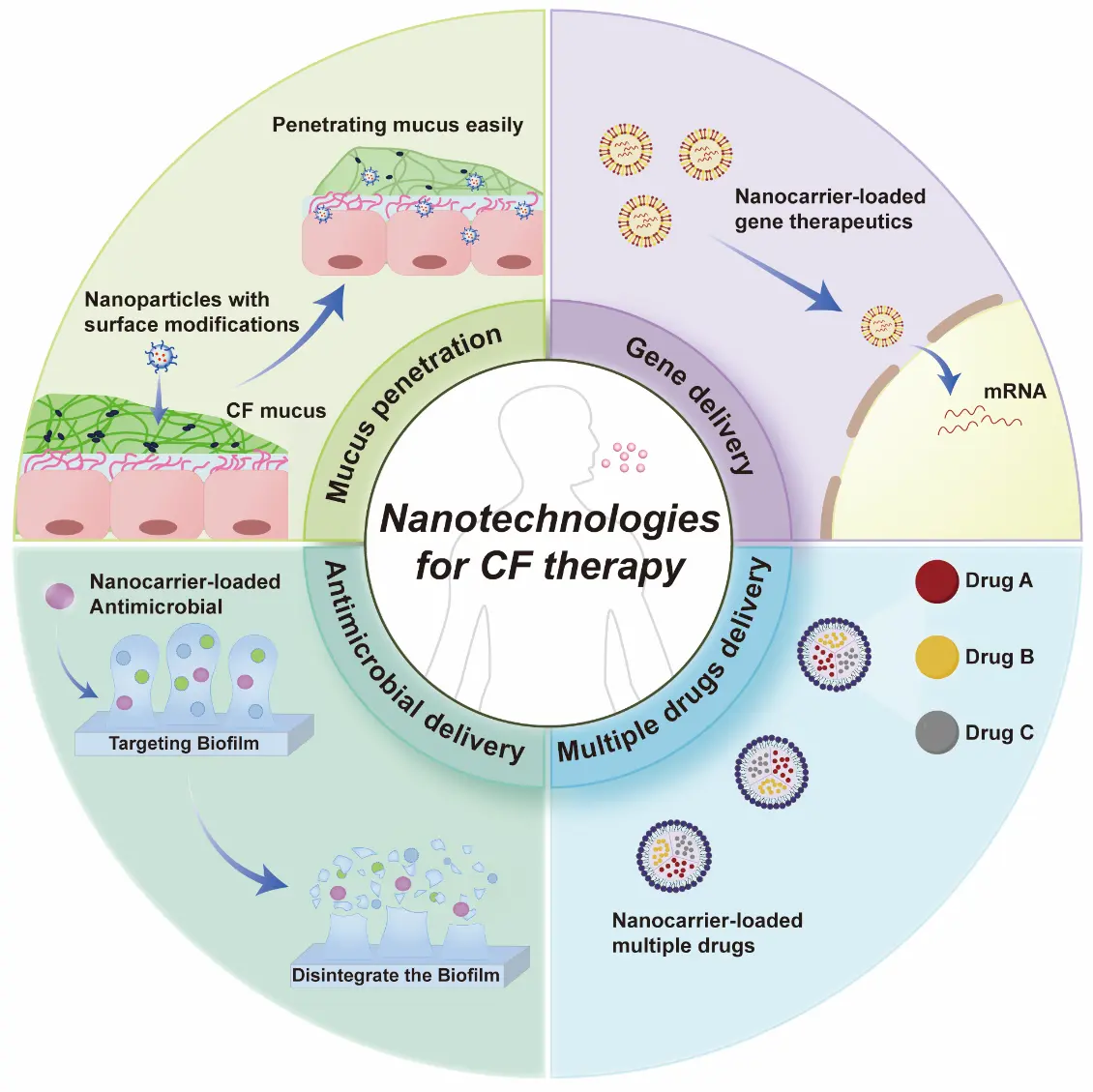
In recent years, nanostructures have shown great promise in the management of CF [6], and nanotechnology offers potential solutions to overcome the aforementioned bottlenecks due to their designable size, surface properties, and targeting capabilities. This opinion paper briefly summarize the latest research advances in this area, which would serve as a reference for the development of nanotherapeutic agents for CF. The main content can be illustrated in Figure 1.
2. Emerging Nanotechnologies
2.1. Mucus Penetration Modification
Highly viscoelastic and adhesive sputum has precluded efficient nanoparticle-based drug delivery to the lungs of patients [7], primarily due to hydrophobic and electrostatic interactions with mucin fibers. To overcome this, nanoparticles are engineered with hydrophilic and charge-neutral surface coatings to minimize mucoadhesion and enhance penetration [8].
In a seminal study, J. Leal, et al. [9] employed phage display technology to screen hydrophilic, near-net-neutral cyclic heptapeptides, which were then covalently conjugated to nanoparticle surfaces. Transport studies revealed that selected mucus-penetrating peptides not only enhanced the bulk diffusion of phage in CF patient sputum by ~600-fold compared to a positively charged control phage clone but also enabled up to 3-fold higher cellular uptake of conjugated nanoparticles compared to non-modified carboxylated and mPEG-conjugated counterparts. This achieves dual enhancement of mucus penetration and epithelial cell uptake [9]. Beyond this approach, S. Hu, et al. [10] have designed poly(lactic-co-glycolic acid) (PLGA) nanoparticles surface-modified with polydopamine (PDA). PDA is a bio-inspired polymer capable of forming a highly hydrophilic and versatile coating that significantly reduces mucoadhesion. This modification achieved not only rapid mucus penetration but also significantly enhanced cellular uptake in vitro and in vivo. In vitro Transwell and agarose gel experiments demonstrated that the mucus penetration rates of PLGA-PDA nanoparticles reached 85.7 ± 1.9% and 69.6 ± 3.4% after 4 h, respectively, significantly higher than those of unmodified PLGA at 57.4 ± 4.3% and 37.2 ± 0.7% (p < 0.001). Compared to traditional PEG or PVA modifications, the PLGA-PDA platform combines stability, biocompatibility, and dual barrier penetration capabilities, demonstrating high conversion potential and promising application prospects. Surface modification strategies for nanoparticles successfully address the challenge of drug penetration through mucus. To classify the diverse physicochemical approaches employed to navigate the CF mucus barrier, we summarize the representative surface engineering strategies and their underlying mechanisms of action in Table 1.
Table 1. Summary of Surface Engineering Strategies for Mucus Penetration [9,10,11].
|
Surface Engineering Strategies for Mucus Penetration |
||
|---|---|---|
|
Strategy |
Representative modification |
Mechanism of Action |
|
Virus-Mimetic Peptide Coating |
L2 Peptide |
Mimics the surface properties of non-enveloped viruses (e.g., HPV) to navigate the mucus mesh without adhesion. |
|
Zwitterionic Surface Modification |
Polydopamine (Zw-PDA) |
Creates a super-hydrophilic hydration shell via equal cationic/anionic charge distribution, preventing mucin adsorption |
|
Dense Hydrophilic Shielding |
PEGylation |
Provides steric hindrance and neutrality to minimize hydrophobic or electrostatic entrapment by mucins. |
As outlined in Table 1, strategies ranging from PEGylation to zwitterionic and peptide modifications collectively aim to minimize electrostatic and hydrophobic entrapment, thereby preventing mucin adhesion and enabling rapid diffusion through CF sputum. However, achieving a fundamental cure for CF requires the targeted delivery of gene therapies to the diseased respiratory epithelial cells, which demands higher precision and biocompatibility from the delivery systems.
2.2. Gene Delivery
Gene therapy holds great promise for cystic fibrosis, as it has the potential to restore the functionality of the CFTR channel by inserting genetic material that codes for the CFTR protein into the cell [12,13]. In particular, mRNA therapy is currently the primary gene therapy for the treatment of CF [14]. However, the delivery of naked nucleic acids is hindered by enzymatic degradation and poor cellular uptake.
To overcome these challenges, lipid nanoparticles (LNPs) have emerged as the “gold standard” for non-viral delivery [15,16]. For instance, X. Bai et al. [15] developed a self-assembly mRNA nanoplatform using a ternary synthetic peptide (peptide 9) and poloxamer T407. In a CF mouse model, the ternary complex showed significantly enhanced mRNA expression in lung tissue compared to binary complexes (T407/mRNA), as substantiated by quantitative bioluminescence imaging. Crucially, the landscape of CF gene therapy is not limited to synthetic carriers. Viral vectors, which can be functionally regarded as “biological nanoparticles”, are also entering the clinical spotlight. Lentiviral vectors have been engineered to pseudotype with envelope proteins (e.g., F/HN proteins) that specifically target lung receptors, thereby enhancing transduction efficiency and lung exclusivity [17]. A landmark first-in-human trial (BI 3720931) is currently evaluating the safety of an inhaled lentiviral vector for CF, marking a significant step toward clinical translation (Table 2).
Table 2. Summary of Key Clinical Trials for CF gene therapies [17,18].
|
Clinical Trials for CF Gene Therapies |
||||||
|---|---|---|---|---|---|---|
|
Therapy |
Vector |
Payload |
Route of Administration |
Sponsor |
NCT |
|
|
BI 3720931 |
Lentiviral Vector |
CFTR DNA |
Nebulized Inhalation |
Boehringer Ingelheim/UK GTC, Bracknell, UK |
NCT06515002 |
|
|
ARCT-032 |
Lipid Nanoparticle |
CFTR mRNA |
Nebulized Inhalation |
Arcturus Therapeutics, Inc., San Diego, CA, USA |
NCT06747858 |
|
|
RCT2100 |
Lipid Nanoparticle |
CFTR mRNA |
Nebulized Inhalation |
ReCode Therapeutics, Menlo Park, CA, USA |
NCT06237335 |
|
|
VX-522 |
Lipid Nanoparticle |
CFTR mRNA |
Nebulized Inhalation |
Vertex Pharmaceuticals Incorporated, Boston, MA, USA |
NCT05668741 |
|
Collectively, these studies reveal a clinical consensus on nebulized inhalation, prioritizing lung exclusivity to minimize systemic exposure. While mRNA-LNPs dominate due to their safety profile, the lentiviral vector BI 3720931 highlights the parallel pursuit of long-term DNA delivery. Thus, next-generation carriers must not only overcome biological barriers but also possess the physical stability to withstand nebulization shear forces for effective transfection.
To summarize, effective gene delivery utilizes advanced nanocarriers to protect genetic payloads and facilitate the functional expression of CFTR in the lungs. Despite the assistance of nanocarriers, rendering gene therapy a highly promising therapeutic regimen for CF, the persistent infectious microenvironment in the lungs of CF patients remains a critical challenge that gene therapy must address.
2.3. Antimicrobial Delivery
Bacterial infections of the lower airways are the main cause of mortality and morbidity in cystic fibrosis [19], particularly due to its biofilm-associated refractory infections [20]. Bacterial biofilms are complex three-dimensional aggregates composed of bacteria and extracellular polysaccharides (EPS). Biofilms not only reduce the effectiveness of antimicrobial treatments, but can also harbor antimicrobial resistant subpopulations, which render their treatment very challenging [21,22]. However, the application of nanotechnology to biofilm eradication has gained significant relevance in recent years. Their small size, penetration efficiency, and design flexibility that they present make them a promising alternative for biofilm infection treatment in people with CF [23]. A notable advancement in this field is exemplified by the study by Al-Momani, et al. [24], who investigated the synergistic efficacy of biosynthesized silver nanoparticles (Ag-NPs) combined with clinically relevant anti-Pseudomonas antibiotics against P. aeruginosa isolates recovered from CF patients. The findings revealed that the MIC of Ag-NPs was 15 μg/mL for all strains which decreased substantially when administered with antibiotics at a dose of 1.875–7.5 μg/mL. The majority of Ag-NP and antibiotic combinations exhibited a synergistic or partially synergistic impact. This was particularly noticeable in combinations containing Meropenem, Ciprofloxacin, and Aztreonam (in which the FIC index was less than or equal to 0.5). Similarly, Garcia Maset, et al. [25] developed a synthetic nano-engineered antimicrobial polymers (SNAPs) which is an ammonium copolymer, named as a-T50 and with a triblock structure. The a-T50 copolymer was synergistic with colistin against planktonic Pseudomonas aeruginosa in synthetic cystic fibrosis medium, and this pair showed a potent synergistic antibiofilm activity against P. aeruginosa in an ex vivo cystic fibrosis pig lung model that it caused a clinically relevant 3-log10 CFU reduction against P. aeruginosa biofilms (p < 0.001). The synergistic combination could also reduce the doses of colistin used in clinics, reducing cytotoxicity while maintaining a potent antibiofilm activity.
In short, these studies underscore that nanotechnology can significantly enhance the therapeutic index of antimicrobial agents. Crucially, the efficacy observed in these physiologically relevant models highlights the potential of nanotechnology for practical implementation via local inhalation. By enabling direct delivery to the lungs, nanocarriers can ensure high local concentrations to penetrate stubborn biofilms while minimizing systemic exposure and toxicity.
2.4. Multiple Drug Delivery
CF patients’ abnormal or absent CFTR protein causes a complex multi-organ disease and clinical symptoms (e.g., accumulation of thick mucus and repeated episodes of infection) [1,3]. Thus, combinatorial therapy such as the co-delivery of antibiotics, CFTR modulators and mucolytics is deemed highly necessary for CF treatment. This integrated approach targets multiple pathological hallmarks of CF simultaneously, thereby optimizing therapeutic efficacy and improving long-term clinical outcomes for CF patients. With the progress that nanotechnology has gained, it enables the co-delivery of different drugs while avoiding drug interactions and improving patient compliance.
Although no such research exists in CF treatment at present, we can leverage insights gained from the field of multi-drug nanomedicine in tumor treatment [26]. For example, W. Ping et al. [27] successfully designed and fabricated luminescent cucurbit-shaped nanoparticles (nanocucurbits) capable of co-encapsulating hydrophilic and hydrophobic chemotherapeutics. This unique architecture enabled the spatiotemporal synchronization of distinct drugs, resulting in potent synergistic antitumor efficacy. While we can draw inspiration from oncology research where co-delivery achieves intracellular synergy, the rationale for CF is unique. It relies on “spatiotemporal synchronization” to overcome sequential biological barriers. For instance, a nanocarrier could facilitate a stage-wise release. First, releasing mucolytics to degrade extracellular mucus, clearing the path for the subsequent delivery of antibiotics to biofilms or gene therapeutics to the epithelium. This strategy addresses the heterogeneity of the lung microenvironment, offering a significant advantage over conventional separate administrations.
In brief, this frontier relies on sophisticated nanoplatforms that can co-encapsulate multiple drugs to achieve synergistic effects against concurrent CF pathologies. And we can infer a key message: the application of nanotechnology in multi-drug delivery represents the culmination of CF treatment approaches and points toward future development directions.
3. Conclusions
Emerging nanotechnologies play an unparalleled role in the treatment of CF. With the assistance of nanotechnology, conventional therapeutic agents have achieved significant enhanced therapeutic efficacy by overcoming key barriers in CF management, such as designing various mucus-penetrating particles (MPPs) and modifying the nanoparticles with different surface modifications to achieve better mucus penetration. Besides, by the use of nanocarriers such as LNPs and polymeric nanoparticles, the stability and capacity for cellular penetration of mRNA delivery have undergone a substantial elevation. To combat recalcitrant bacterial infections, a nanocarrier-mediated delivery of antimicrobial agents enables efficient disruption of obstacles, including biofilms, and consequently enhances the therapeutic efficacy. Furthermore, based on existing multi-drug nanomedicine for other diseases, we can infer that multi-drug delivery via nanocarrier-based systems holds substantial promise for cystic fibrosis therapy and will undoubtedly be realized. In the future, the integration of these nanoparticles with pulmonary drug delivery devices for the development of inhalable formulations, the realization of industrial-scale production of these nanoformulations, and the accelerated advancement of clinical trials will hold profound clinical significance. This opinion paper provided valuable insights for the development of novel CF therapies and innovative nanoformulations.
Author Contributions
Conceptualization, X.Z. and Z.H.; Investigation, D.P.; Writing—Original Draft Preparation, D.P.; Writing—Review & Editing, X.Z. and Z.H.; Supervision, X.Z. and Z.H.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Funding
This work was supported by Guangdong Natural Science Foundation (No.2024A1515010896) and Youth S&T Talent Support Program of Guangdong Provincial Association for Science and Technology (Grant No.SKXRC2025338).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
Mall MA, Burgel PR, Castellani C, Davies JC, Salathe M, Taylor-Cousar JL. Cystic fibrosis. Nat. Rev. Dis. Primers. 2024, 10, 53. doi:10.1038/s41572-024-00538-6. [Google Scholar]
-
Polgreen PM, Comellas AP. Clinical Phenotypes of Cystic Fibrosis Carriers. Annu. Rev. Med. 2022, 73, 563–574. doi:10.1146/annurev-med-042120-020148. [Google Scholar]
-
Grasemann H, Ratjen F. Cystic Fibrosis. N. Engl. J. Med. 2023, 389, 1693–1707. doi:10.1056/NEJMra2216474. [Google Scholar]
-
Jia SJ, Taylor-Cousar JL. Cystic Fibrosis Modulator Therapies. Annu. Rev. Med. 2023, 74, 413–426. doi:10.1146/annurev-med-042921-021447. [Google Scholar]
-
Graeber SY, Mall MA. The future of cystic fibrosis treatment: from disease mechanisms to novel therapeutic approaches. Lancet 2023, 402, 1185–1198. doi:10.1016/S0140-6736(23)01608-2. [Google Scholar]
-
George M, Boukherroub R, Sanyal A, Szunerits S. Treatment of lung diseases via nanoparticles and nanorobots: Are these viable alternatives to overcome current treatments? Mater. Today Bio. 2025, 31, 101616. doi:10.1016/j.mtbio.2025.101616. [Google Scholar]
-
Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP, et al. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–2597. doi:10.1016/j.biomaterials.2008.12.076. [Google Scholar]
-
Zhao J, Qin L, Song RX, Su J, Yuan Y, Zhang X, et al. Elucidating inhaled liposome surface charge on its interaction with biological barriers in the lung. Eur. J. Pharm. Biopharm. 2022, 172, 101–111. doi:10.1016/j.ejpb.2022.01.009. [Google Scholar]
-
Leal J, Peng XJ, Liu XQ, Arasappan D, Wylie DC, Schwartz SH, et al. Peptides as surface coatings of nanoparticles that penetrate human cystic fibrosis sputum and uniformly distribute in vivo following pulmonary delivery. J. Control. Release 2020, 322, 457–469. doi:10.1016/j.jconrel.2020.03.032. [Google Scholar]
-
Hu SS, Yang ZX, Wang S, Wang LP, He QQ, Tang H, et al. Zwitterionic polydopamine modified nanoparticles as an efficient nanoplatform to overcome both the mucus and epithelial barriers. Chem. Eng. J. 2022, 428, 132107. doi:10.1016/j.cej.2021.132107. [Google Scholar]
-
Huckaby JT, Lai SK. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. doi:10.1016/j.addr.2017.08.010. [Google Scholar]
-
Christopher Boyd A, Guo SL, Huang LL, Kerem B, Oren YS, Walker AJ, et al. New approaches to genetic therapies for cystic fibrosis. J. Cyst. Fibros. 2020, 19, S54–S59. doi:10.1016/j.jcf.2019.12.012. [Google Scholar]
-
Maldonado I, Gallego I, Enriquez-Rodriguez L, Ibarra AI, Arbe A, Mashal M, et al. A novel gene delivery approach to face cystic fibrosis by non-viral vectors based on niocarbosomes. Surf. Interf. 2025, 68, 106639. doi:10.1016/j.surfin.2025.106639. [Google Scholar]
-
Zhang MJ, Lu HY, Xie LK, Liu XL, Cun DM, Yang MS. Inhaled RNA drugs to treat lung diseases: Disease-related cells and nano–bio interactions. Adv. Drug Deliv. Rev. 2023, 203, 115144. doi:10.1016/j.addr.2023.115144. [Google Scholar]
-
Bai X, Chen QJ, Li FQ, Teng YL, Tang MP, Huang J, et al. Optimized inhaled LNP formulation for enhanced treatment of idiopathic pulmonary fibrosis via mRNA-mediated antibody therapy. Nat. Commun. 2024, 15, 6844. doi:10.1038/s41467-024-51056-8. [Google Scholar]
-
Zhang KQ, Zhou Y, Wang GL, Zhu B, Zhao ZY, Kong X, et al. Pulmonary mRNA delivery systems for the treatment of respiratory diseases: Current advances and challenges. Chin. Chem. Lett. 2025, 36, 111887. doi:10.1016/j.cclet.2025.111887. [Google Scholar]
-
Davies JC, Polineni D, Boyd AC, Donaldson S, Gill DR, Griesenbach U, et al. Lentiviral gene therapy for cystic fibrosis: A promising approach and first-in-human trial. Am. J. Respir. Crit. Care Med. 2024, 210, 1398–1408. doi:10.1164/rccm.202402-0389CI. [Google Scholar]
-
Gill NA, Fung V, VanKeulen-Miller R, Narasipura EA, Fenton OS. Non-viral mRNA cystic fibrosis therapies and their ongoing clinical trials. Expert Opin. Drug Deliv. 2025. doi:10.1080/17425247.2025.2586172. [Google Scholar]
-
Taccetti G, Terlizzi V, Campana S, Dolce D, Ravenni N, Fevola C, et al. Antibiotic treatment of bacterial lung infections in cystic fibrosis. Eur. J. Pediatr. 2024, 184, 82. doi:10.1007/s00431-024-05905-9. [Google Scholar]
-
Zou Y, Liang CM, Tang ZQ, Huang YT, Liang CF, Chen LM, et al. Biofilm-Responsive Nano-Antibiotics for Degradation of Extracellular Polymeric Substance Matrix and Reduction of Pathogenicity against Drug-Resistant Bacterial Infections. ACS Nano. 2025, 19, 29702–29716. doi:10.1021/acsnano.5c09764. [Google Scholar]
-
Zhou Y, Huang JY, Wang GL, Zhai ZZ, Ahmed MU, Xia X, et al. Polymyxin B sulfate inhalable microparticles with high-lectin-affinity sugar carriers for efficient treatment of biofilm-associated pulmonary infections. Sci. Bull. 2023, 68, 3225–3239. doi:10.1016/j.scib.2023.11.004. [Google Scholar]
-
Qin SG, Xiao W, Zhou CM, Pu QQ, Deng X, Lan LF, et al. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. doi:10.1038/s41392-022-01056-1. [Google Scholar]
-
Blanco-Cabra N, Alcàcer-Almansa J, Admella J, Arévalo-Jaimes BV, Torrents E. Nanomedicine against biofilm infections: A roadmap of challenges and limitations. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1944. doi:10.1002/wnan.1944. [Google Scholar]
-
Al-Momani H, Albalawi H, Al Balawi D, Khleifat KM, Aolymat I, Hamed S, et al. Enhanced Efficacy of Some Antibiotics in the Presence of Silver Nanoparticles Against Clinical Isolate of Pseudomonas aeruginosa Recovered from Cystic Fibrosis Patients. IJN 2024, 19, 12461–12481. doi:10.2147/IJN.S479937. [Google Scholar]
-
Maset RG, Hapeshi A, Lapage J, Harrington N, Littler J, Perrier S, et al. Combining SNAPs with antibiotics shows enhanced synergistic efficacy against S. aureus and P. aeruginosa biofilms. Npj Biofilms Microbiomes 2023, 9, 36. doi:10.1038/s41522-023-00401-8. [Google Scholar]
-
Benderski K, Lammers T, Sofias AM. Analysis of multi-drug cancer nanomedicine. Nat. Nanotechnol. 2025, 20, 1163–1172. doi:10.1038/s41565-025-01932-1. [Google Scholar]
-
Wei P, Ding YS, Liu SN, Jiang JH, Chen JH. Luminescent Nanocucurbits Enable Spatiotemporal Co-Delivery of Hydrophilic and Hydrophobic Chemotherapeutic Agents. Adv. Sci. 2025, 12, e09782. doi:10.1002/advs.202509782. [Google Scholar]