Electric energy storage devices are essential electronic components in fields such as advanced microelectronics, electric vehicles, and aerospace [
1]. Among them, dielectric capacitor, which stores electrical energy in the form of electrostatic fields, are widely used, resulting from their rapid charging and discharging speed, eminent power density, and excellent reliability [
2]. Dielectric capacitors can typically be divided into ceramic capacitors and polymer thin film capacitors [
3]. The ceramic dielectric materials have relatively high dielectric constant, but they also exhibit disadvantages such as low breakdown strength, high processing temperature, and brittleness [
4]. With the development of capacitors towards miniaturization and lightweight, More and more attention has been paid to polymeric dielectric capacitors [
5,
6,
7,
8]. Polymer dielectrics not only have the advantages of lightweight, easy processing, and high breakdown strength but also demonstrate many unique advantages, covering low price, mechanical flexibility, and strong charge-discharge cycling ability [
9]. However, the relatively low operating temperature of polymers limits their further applications. For example, the widely used biaxially oriented polypropylene (BOPP) exhibits ultra-high breakdown strength at room temperature, but its breakdown strength sharply decreases when the working temperature exceeds 105 °C, which cannot meet the high-temperature requirements of aerospace, hybrid vehicles, oil and gas exploration and other fields [
10]. Therefore, it is urgent to develop polymer based dielectric materials with excellent energy storage performance to meet the high-temperature application needs of electronic power systems.
In order to meet the operation requirements of contemporary high-power electronic devices at high temperatures, a series of super engineering plastics showing high glass transition temperature (
Tg) have been developed [
11,
12]. Resulting from the abundant aromatic groups or condensed heterocyclic rings on the main chain, these polymers have high thermal and mechanical stability [
13]. Typical representatives include phenylene sulfide (PPS) [
14], poly(arylene ether ketone) (PEK) [
15], poly(arylene ether nitrile) (PEN) [
16], poly(arylene ether urea) (PEEU) [
17], polyetherimide (PEI) [
18,
19], polyimide (PI) [
20],
etc. [
21,
22,
23]. Among them, PEN is a structurally regular linear semi-crystalline polymer with cyanide groups at the side chain and aromatic ether bonds in the main chain [
24]. The aromatic ether bond in its main chain enables it to reach a
Tg higher than 150 °C. On the other hand, the presence of cyanide endows it with a high dielectric constant, a prerequisite for high energy storage density [
25]. Additionally, the cyanide group at the side chain of PEN also gives it excellent processing and molding ability. Compared to materials such as PI, PEN can be melted or solution processed, which demonstrates promising application in fields such as military defense, the microelectronics industry, electronic circuits, automobile manufacturing, aircraft manufacturing, and petrochemicals [
26].
Crosslinking is another effective method to improve the thermal stability of polymer dielectrics [
27]. On one hand, it restricts the movement and polarization of polymer segments, thus enhancing thermal stability [
28]. On the other hand, it elevates breakdown strength, thereby improving the energy storage performance [
29]. Most crosslinking processes require the addition of crosslinking agents, while the residue crosslinking agents would depress the energy storage performance. In addition, the released gas and/or small molecule substances during crosslinking could also affect the energy storage performance [
30,
31]. Therefore, a suitable crosslinking reaction is important for preparing high-temperature dielectric materials. Accordingly, Zhang and coauthors [
32] fabricated a series of crosslinked PEI having different crosslinking degrees by adjusting the crosslinking reaction of phenylacetylene groups. When the gel content reaches 35 wt%, and the molecular weight of the soluble chain is about 6 × 10
4, the discharged energy density exceeds 3.60 J/cm
3 at 150 °C with an efficiency that remains above 95%. Our previous researches show that phthalonitriles can undergo self-crosslinking reactions at high temperatures, forming phthalocyanine rings without releasing small molecules [
33,
34]. Basing on this reaction, high-temperature dielectrics with a
Tg higher than 350 °C have been successfully prepared from phthalonitriles modified BN and CNT, as well as phthalonitriles capped PEN [
35].
In this work, TiO
2 and PEN hybrid materials are prepared via the self-crosslinking of phthalonitriles, and act as high-temperature dielectrics. To improve the thermal stability of these dielectrics, a cross-linkable PEN terminated with phthalonitriles is first prepared. Subsequently, phthalonitriles modified TiO
2 is introduced into the cross-linkable PEN matrix by grafting phthalonitriles on the surface of TiO
2. After self-crosslinking at high temperatures, TiO
2-PEN hybrids are successfully obtained. Results show that the TiO
2-PEN hybrids can be used as a high-temperature dielectrics due to their long-term use at 150 °C.
2.1. Materials
TiO
2 (20–30 nm) was provided by Tianjin Kemier. 4-Nitrophthalonitrile (4-NPh) biphenyldiol (BP) and dichlorobenzonitrile (DCBN), and hydroquinone (HQ) were purchased from Shanghai Macklin Biochemicals. Hydrogen peroxide (H
2O
2), N-methylpyrrolidone (NMP) and N,N-dimethylformamide (DMF) were obtained from local suppliers. PEN terminated with phthalonitriles was synthesized according to the reference [
36]. Other chemicals were commercially available products and used directly.
2.2. Synthesis of Phthalonitriles Modified TiO2 (TiO2-2CN)
TiO
2-2CNwas synthesized according to the literature [
37]. Specifically, 5.0 g TiO
2 was added into H
2O
2 (0.25 L), then heated to reflux for 4 h upon stirring. After centrifugal separation and washing with deionized water for 3 times, TiO
2-OH was synthesized via drying at 80 °C for 12 h in a vacuum. This obtained TiO
2-OH (1.0 g) and 4-NPh (1.0 g) were added into DMF (250 mL) with the existence of potassium carbonate (1.0 g). This blend was heated to reflux with stirring overnight. After the reaction, the crude product was centrifuged and then washed with DMF and water each for 3 times. TiO
2-2CN was finally obtained via drying at 100 °C overnight in a vacuum.
2.3. Preparation of TiO2/PEN Nanocomposites
The TiO
2/PEN nanocomposites were prepared by using the solution casting method. Firstly, a certain amount of TiO
2-2CN and PEN-2CN were added to 10 mL of NMP, and stirred at 60 °C for 2 h. The obtained homogeneous mixture was then cast onto a clean glass plate and heated in a program-controlled blast oven. After removing the solvent, TiO
2/PEN nanocomposite films with a thickness of approximately 50 μm were separated from the glass plate. TiO
2/PEN nanocomposite films with TiO
2-2CN contents of 0, 5, 10, 15, and 20 wt% were prepared by controlling the dosage of TiO
2-2CN and PEN-2CN.
2.4. Fabrication of TiO2-PEN Hybrids
TiO
2-PEN hybrid films were fabricated via the self-crosslinking reaction of phthalonitriles by treating the TiO
2/PEN nanocomposite film in a 320 °C muffle furnace for 4 h. Basing on the content of TiO
2-2CN in TiO
2/PEN nanocomposites, the hybrids were named 0, 5, 10, 15, and 20 wt% TiO
2-PEN, respectively.
2.5. Characterization
FTIR spectra of TiO
2, 4-NPh and TiO
2-2CN were collected using a Nicolet 200SXV instrument (Thermofisher, Madison, WI, USA). The crystalline structures of TiO
2 and TiO
2-2CN were measured by D8 Advance XRD (Bruker, Karlsruhe, Germany) using Cu Kα radiation. XPS (Escalab 250xi, Thermofisher, Madison, WI, USA) with an Al Kα X-ray source was used to test the band structure of TiO
2-2CN. Micro-morphology of TiO
2, nanocomposite films and hybrid films were tested using TESCAN MIRA4 (Brno, Czech) field emission scanning electron microscopy (SEM) with an acceleration voltage of 15 kV. Dielectric performance was tested using a TH2826 LCR meter (Tong Hui, Changzhou, China). Breakdown strength of the samples was tested with a ZJC-5 kV withstand voltage tester (Zhonghang, Beijing, China). The thermal properties of the samples were obtained by TGA (HTG-1, Beijing Hengjiu, Beijing, China) and DSC (DSC-1, Beijing Hengjiu, Beijing, China) in a nitrogen environment. The polarization electric field (P-E) loops of TiO
2/2CN and TiO
2-2CN were measured using a ferroelectric tester (Premiere II, Radiant Technologies, Alpharetta, GA, USA) at a frequency of 1000 Hz. The simulation of the breakdown process of the thin film was conducted via finite element analysis using COMSOL Multiphysics 6.2, with detailed information in the Supplementary Materials.
In this study, high-temperature dielectrics TiO
2-PEN hybrids are fabricated through self-crosslinking reaction of phthalonitriles from TiO
2-2CN and PEN-2CN. Firstly, PEN-2CN terminated with phthalonitriles (
a) is prepared. Simultaneously, phthalonitrile groups are grafted onto the surface of TiO
2 to obtain TiO
2-2CN (
b). TiO
2/PEN nanocomposites are then prepared from TiO
2-2CN and PEN-2CN using the solution casting method. Finally, the nanocomposites TiO
2/PEN are treated at high temperatures to induce self-crosslinking reaction of phthalonitriles, resulting in the high-temperature dielectrics TiO
2-PEN hybrids (
c).
PEN-2CN was prepared according to the literature [
36]. TiO
2-2CN was synthesized via a two-step procedure, which includes the hydroxylation of TiO
2 and a reaction with 4-NPh [
37].
a shows the FTIR spectra of TiO
2, 4-NPh and TiO
2-2CN. TiO
2 exhibits infrared absorption peaks at 3435 and 1628 cm
−1, which are derived from crystallization water and hydroxyl groups at the periphery of TiO
2, respectively [
38]. As an organic compound, 4-NPh exhibits a series of peaks, including at 2227 cm
−1 (cyano group), 1618–1514 cm
−1 (benzene ring) and 1352 cm
−1 (C-H) on benzene [
39]. After the two-step reaction, the absorption peak of TiO
2-2CN at 3435 cm
−1 becomes wider, indicating that there are more hydroxyl groups that are formed during the hydroxylation process. In addition, peaks appear at 2227, 1628, 1352, and 1299 cm
−1 suggesting that phthalonitriles have been grafted onto its surface.
b is the TGA curves of TiO
2 and TiO
2-2CN. TiO
2 exhibits a mass reduction of less than 2.0 wt% from 80–800 °C, which would be due to the loss of surface crystalline water. In contrast, the residual mass of TiO
2-2CN at 800 °C is only 90.6 wt%. Therefore, it can be calculated that the grafting ratio of phthalonitriles on TiO
2-2CN is 7.6 wt% [
40].
. (<b>a</b>) Structure of PEN-2CN; (<b>b</b>) preparation procedures for TiO<sub>2</sub>-2CN; (<b>c</b>) scheme for the fabrication of TiO<sub>2</sub>-PEN hybrids.
. (<b>a</b>) FTIR spectra, (<b>b</b>) TGA curves and (<b>c</b>) XRD patterns of TiO<sub>2</sub> and TiO<sub>2</sub>-2CN; (<b>d</b>) XPS, (<b>e</b>) XPS C1s and (<b>f</b>) XPS N1s spectrum of TiO<sub>2</sub>-2CN.
The composition of TiO
2-2CN was also characterized via XPS. As displayed by
d, the XPS full spectrum of TiO
2-2CN displays elements including O (530.0 eV), Ti (460.0 eV), N (402.2 eV), and C (285.0 eV), respectively, indicating the existence of organic and inorganic components. The XPS C1s spectrum can be further decomposed into peaks at 288.6, 286.1 and 284.5 eV, corresponding to -C≡N, C-O, and C-C, respectively (
e) [
41]. In addition, the XPS N1s spectrum shows a single -C≡N peak at 399.6 eV (
f) [
42]. The XPS results are consistent with the structure of TiO
2-2CN, indicating the successful grafting of phthalonitriles. Finally, the structure of TiO
2-2CN was confirmed by XRD. As depicted in
c, TiO
2 demonstrates diffraction peaks at 25.28, 37.80, 48.05, 53.89, 55.06, 62.69, 68.87, 70.39, 74.15, 75.15, and 76.12
o, corresponding to crystal planes of (101), (004), (200), (105), (211), (204), (116), (220), and (215), respectively. This result is consistent with the published standard card of JCPDS No. 21-1272, indicating an anatase type TiO
2 [
43]. In contrast, the XRD diffraction peaks of TiO
2-2CN become wider after grafting of phthalonitriles, while their position is maintained, suggesting the same crystalline structure of TiO
2.
The micro-morphology of TiO
2 and TiO
2-2CN was further studied by SEM and TEM observation.
a,b shows SEM images of TiO
2, revealing numerous individual particles with a particle size of approximately 20 nm. In contrast, after surface modification, TiO
2-2CN appears to be stuck together with similar particle sizes (
e). In addition,
f is the SEM mapping images of TiO
2-2CN. C, N, Ti and O elements can be clearly identified from these figures.
c,d is TEM images of TiO
2 and TiO
2-2CN. The blurred boundaries on the surface of TiO
2-2CN particles indicate that their surfaces have been modified. All of the above results indicate that TiO
2-2CN has been successfully prepared. Subsequently, the synthesized TiO
2-2CN was incorporated into PEN-2CN to prepare TiO
2/PEN nanocomposites, which transform into TiO
2-PEN hybrids afterwards.
g shows the cross-section morphology of PEN-2CN, from which some fracture marks can be observed, indicating its tough nature [
44]. The fracture marks scale up and form fish-scale-like cracks after the incorporation of TiO
2-2CN (
i). Moreover, TiO2-2CN is homogeneously dispersed in the polymer matrix even at the filler content of 20 wt%. This would be resulted from the surface modification of TiO
2-2CN, which exhibits excellent compatibility with PEN, and can be confirmed by the corresponding SEM mapping images in
j [
45].
h is the SEM cross-section morphology of PEN-2CN after self-crosslinking (0 wt% TiO
2-PEN). Compared to that of PEN-2CN, the cross-section is denser and neater, suggesting a brittle fracture. The brittle fracture is caused by the self-crosslinking reaction of phthalonitriles in PEN-2CN, which transforms the system from a thermoplastic system to a thermosetting network [
46]. Similar phenomena are also observed in the cross-sectional view of the other hybrids containing fillers. For example, no fish-scale-like crack is observed in the hybrid 10 wt% TiO
2-PEN, but there is a brittle fracture morphology (
k). Additionally, compared with the plenty of TiO
2-2CN particles observed aside from the fish-scale-like cracks in 10 wt% TiO
2/PEN, there is not any obvious nanofiller that can be seen from the cross-section of 10 wt% TiO
2-PEN. The result is a consequence of the formed covalent bonds between TiO
2 and PEN. As the organic part is easier to break during liquid nitrogen brittling, TiO
2 would be encapsulated beneath the cross-section. Nevertheless, the TiO
2-PEN hybrid still demonstrates excellent flexibility and can be cyclically bent without breaking, as depicted in
l.
. SEM (<b>a</b>,<b>b</b>) and TEM (<b>c</b>) images of TiO<sub>2</sub>; TEM (<b>d</b>), SEM (<b>e</b>) and SEM mapping images (<b>f</b>) of TiO<sub>2</sub>-2CN; SEM image of 0 wt% TiO<sub>2</sub>/PEN (<b>g</b>), 0 wt% TiO<sub>2</sub>-PEN (<b>h</b>), 10 wt% TiO<sub>2</sub>/PEN (<b>i</b>) and 10 wt% TiO<sub>2</sub>-PEN (<b>k</b>); SEM mapping images of 10 wt% TiO<sub>2</sub>/PEN (<b>j</b>); (<b>l</b>) photographs of 10 wt% TiO<sub>2</sub>/PEN and 10 wt% TiO<sub>2</sub>-PEN.
The transition of TiO
2/PEN nanocomposites into TiO
2-PEN hybrids can also be confirmed by their thermal and mechanical properties.
Figure S1 shows the DSC heating curves of TiO
2/PEN nanocomposites. The
Tg of 0 wt% TiO
2/PEN is 195.4 °C, and the
Tg increases gradually as the filler content augments to 20 wt%. For comparison, the
Tg of TiO
2-PEN hybrids is higher than that of TiO
2/PEN nanocomposite. This is because these TiO
2-PEN hybrids are cross-linked networks in which the movement of polymer segments is restricted (
Figure S2) [
47]. Similar results can also be obtained from the TGA curves of TiO
2/PEN and TiO
2-PEN. As shown in
Figure S3 and
Figure S4, the residual mass of 0 wt% TiO
2/PEN at 800 °C is 60.1 wt%, while it is 66.3 wt% for 0 wt% TiO
2-PEN due to its crosslinked structure. Naturally, attributing to the relatively high residual mass of TiO
2-2CN, the residual mass of both TiO
2/PEN and TiO
2-PEN at 800 °C increases gradually. Moreover, the crosslinking of TiO
2-PEN hybrids is also certified by their augmented thermal decomposition temperature (
T10%). The
T10% of TiO
2-PEN hybrids is not only higher than that of TiO
2/PEN nanocomposites but also increases obviously with the increase of filler content (
Table S1 and
Table S2). With the increasing of TiO
2-2CN content, the number of phthalonitrile groups that can undergo self-crosslinking reaction increases, which leads to an augment in crosslinking density in TiO
2-PEN hybrids and thus enhances their
T10% [
48].
Figure S5,
Figure S6,
Figure S7,
Figure S8,
Figure S9 and
Figure S10 are the mechanical properties of TiO
2/PEN and TiO
2-PEN. Compared with TiO
2/PEN nanocomposites, TiO
2-PEN hybrids demonstrate higher tensile strength and modulus but lower elongation at break at the same filler content, which is consistent with the fact that TiO
2-PEN is self-crosslinked from TiO
2/PEN. Interestingly, the tensile strength and modulus of 5 wt% TiO
2-PEN reach the crest values of 115.3 MPa and 2277 MPa, which can be explained from two aspects: on one hand, the tensile strength and modulus are improved after crosslinking, and the amount of improvement is affected by the crosslinking density; on the other hand, at the increased filler concentration, both of tensile strength and modulus decrease gradually. The combination of these effects results in the peak values at the filler content of 5 wt% [
49].
After the preparation of TiO
2/PEN and TiO
2-PEN, their dielectric properties were investigated in detail.
a exhibits the dielectric constant variation of TiO
2/PEN with frequency. Firstly, it can be seen that the dielectric constant of TiO
2/PEN increases gradually as TiO
2 content increases. Specifically, at 1 kHz, it increases from 3.86 for 0 wt% TiO
2/PEN to 4.72 for 20 wt% TiO
2/PEN (
Table S1). Moreover, the dielectric constant of each TiO
2/PEN composite decreases with the increased frequency. This could be explained by the effect of polarization relaxation, which is always observed in the system with higher filler content [
50]. Nevertheless, the dielectric constant of TiO
2/PEN is stable as the highest variation of dielectric constant with frequency is just 3.7 × 10
−7 °C
−1 from 100 Hz to 1 MHz. Similar phenomena are also obtained for the dielectric constant of TiO
2-PEN (
b). However, due to the crosslinked system, the dielectric constant and its variation values are lower than those of TiO
2/PEN at the same filler concentration, as demonstrated by
c. Dielectric loss of TiO
2/PEN and TiO
2-PEN exhibit the same result as that of dielectric constant (
Figure S11 and
Figure S12), as reported in the literature [
50].
d depicts the Weibull distribution of the breakdown strength of TiO
2/PEN [
51]. It is obvious that the breakdown strength of TiO
2/PEN decreases gradually as the filler content increases. This is a results of the interface incompatibility between inorganic fillers and polymer matrix, which exacerbates the distortion of the electric field. In order to further investigate the breakdown process of dielectrics, finite element analysis based on COMSOL Multiphysics 6.2 was conducted. The calculation adopts the phase field dielectric breakdown model developed by Hong and coworkers, which is described in the supporting information [
52].
demonstrates the simulated breakdown process of PEN-2CN, 10 wt% TiO
2/PEN, and 10 wt% TiO
2-PEN at different times, respectively. The formation process of the electric tree can be easily recognized from the figures. Comparing with pure PEN-2CN, the breakdown strength of TiO
2/PEN decreases with the increase of filler content due to the fact that the interfaces between TiO
2 and PEN are easier to break down. In addition, the electric breakdown tree of 10 wt% TiO
2-PEN is bigger than that of 10 wt% TiO
2/PEN at 4 s, which is consistent with the result of the breakdown strength shown in
e. There are literature reports that the breakdown strength increases after crosslinking [
53]. Consequently, the decreasing breakdown strength of TiO
2-PEN from TiO
2/PEN could be attributed to the formation of conjugated phthalocyanine rings from the self-crosslinking of phthalonitriles. The conjugated phthalocyanine rings can serve as conductive points in the system, which is suitable for the passing of electric trees and results in the abating of the breakdown strength of TiO
2-PEN (
f) [
54]. The P-E loops of TiO
2/PEN and TiO
2-PEN were further tested to investigate their energy storage performance. The maximum electric field of the P-E loops was set to be 180 kV/mm, due to the 194.3 kV/mm breakdown strength of 20 wt% TiO
2-PEN (
g,h). According to the P-E loops, it can be concluded that both TiO
2/PEN and TiO
2-PEN are linear dielectrics [
55]. Additionally, the discharged energy density (
Ud,
i) and efficiency (
η,
Figure S13) of TiO
2/PEN and TiO
2-PEN can be obtained via integrating these lines (
Table S1 and
Table S2) [
55]. With the increase of TiO
2-2CN content,
Ud of both TiO
2/PEN and TiO
2-PEN increases. Basing on literature,
Ud of linear dielectrics is related to their dielectric constant and breakdown strength [
56]. Thereby, the increased
Ud is determined by the increased dielectric constant as the electric field strength is 180 kV/mm. In addition, it can be obtained from
Table S2 that
Ud of TiO
2-PEN is higher than that of TiO
2/PEN at filler content higher than 10 wt%. This phenomenon could be a result of the higher growth rate of their dielectric constant. The efficiencies of both TiO
2/PEN and TiO
2-PEN decrease with the increment of filler content, the same as that of their dielectric loss (
Figure S13). Fortunately, the efficiency is still greater than 90%, even at the TiO
2-2CN content of 20 wt%.
. (<b>a</b>–<b>c</b>) Dielectric constant of TiO<sub>2</sub>/PEN and TiO<sub>2</sub>-PEN; (<b>d</b>–<b>f</b>) breakdown strength of TiO<sub>2</sub>/PEN and TiO<sub>2</sub>-PEN; (<b>g</b>,<b>h</b>) P-E curves and (<b>i</b>) discharged energy density of TiO<sub>2</sub>/PEN and TiO<sub>2</sub>-PEN.
. Finite element analysis of the nominal electric potential distribution at various moments during breakdown for different samples. (<b>a</b>) PEN-2CN; (<b>b</b>) 10 wt% TiO<sub>2</sub>/PEN and (<b>c</b>) 10 wt% TiO<sub>2</sub>-PEN.
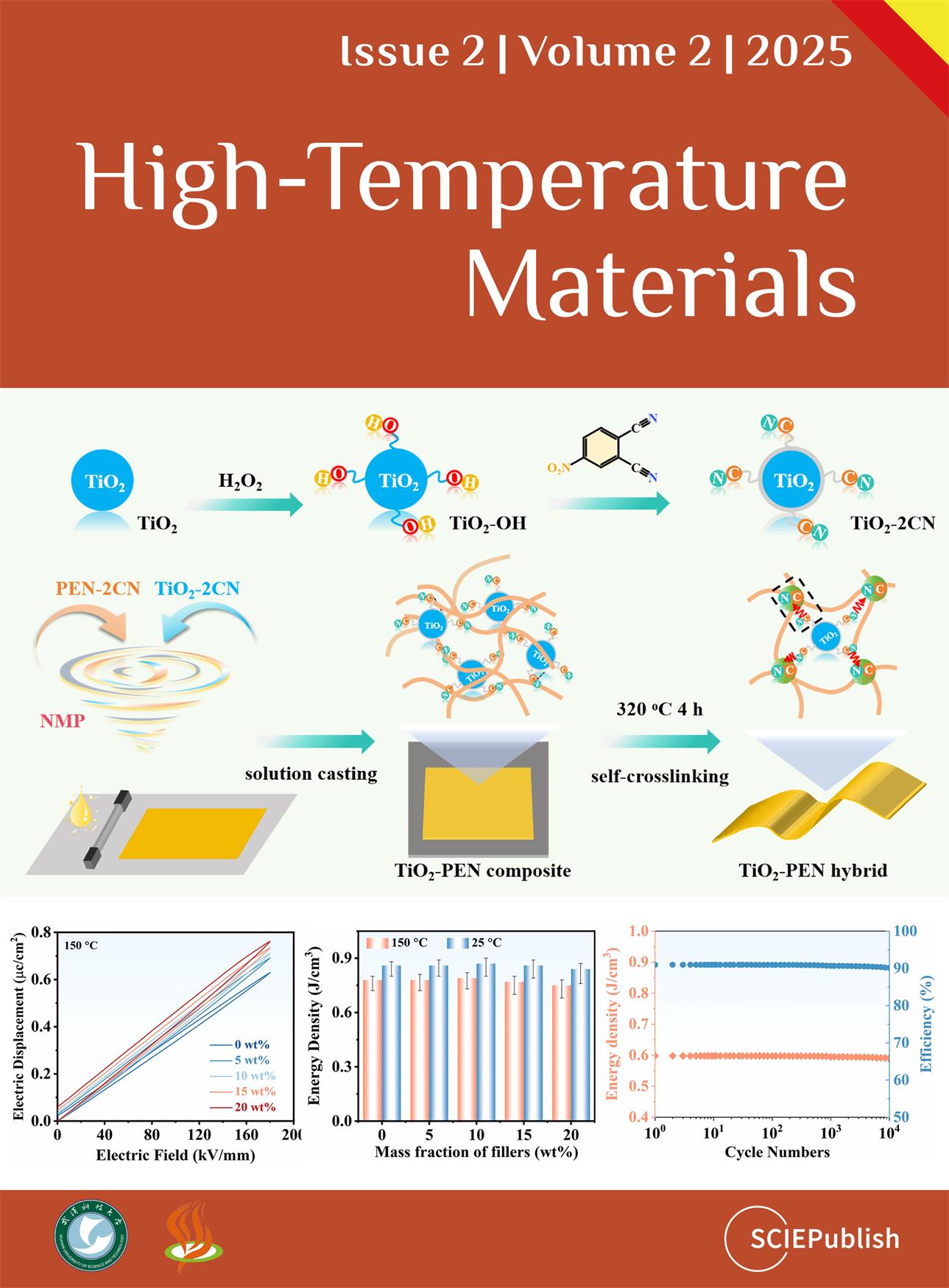
Finally, the high-temperature dielectric properties of TiO
2-PEN were detailedly studied.
a exhibits the dielectric constant of 10 wt% TiO
2-PEN at different temperatures. Similar to the result at room temperature, the dielectric constant of TiO
2-PEN decreases with increasing frequency at high temperature. In addition, as the temperature ascends, TiO
2-PEN dielectric constant at the same frequency also increases gradually. Typically,
b displays the variation of dielectric constant of TiO
2-PEN from room temperature to 200 °C at 1 kHz. This enhanced dielectric constant can be attributed to the higher mobility of chain segments and/or polar groups at higher temperatures, which can strengthen the polarization of the system [
57]. Nevertheless, this TiO
2-PEN hybrid demonstrates excellent dielectric constant stability within the temperature range as the variation is just 2.7 × 10
−7 Hz
−1.
c,d reveals the changes of dielectric loss of 10 wt% TiO
2-PEN at different temperatures, which shows the same phenomena as that of the dielectric constant. The dielectric properties of other TiO
2-PEN hybrids at different temperatures present similar results, as displayed in
Figure S14,
Figure S15,
Figure S16,
Figure S17,
Figure S18,
Figure S19,
Figure S20 and
Figure S21. Furthermore, the breakdown strength and P-E loops of TiO
2-PEN were typically tested at 150 °C. At 150 °C, the breakdown strength of TiO
2-PEN decreases with the increment of TiO
2-2CN (
e). Moreover, the P-E loops are still linear, while the hysteresis area, representing the loss of energy density, in these loops, becomes larger than that at room temperature (
g). This can be explained by the increased dielectric loss at high temperatures, which consumes part of the charged energy and converts it into heat. According to the P-E loops, the
Ud of TiO
2-PEN at 150 °C is calculated to be 0.54, 0.59, 0.60, 0.61 and 0.62 J/cm
3 when TiO
2-2CN is raised from 0 to 20 wt% (
i), while the efficiency remains above 85%. Although the
Ud of 10 wt% TiO
2-PEN abates with the increased temperature, it maintained 95% of its value at room temperature (0.62 J/cm
3), suggesting excellent thermal stability. In addition, the energy density of TiO
2-PEN at 150 °C could be as high as 0.77, 0.78, 0.79, 0.77, and 0.74 J/cm
3 for 0, 5, 10, 15, and 20 wt% TiO
2-PEN (
h) basing on the calculation from their dielectric constant and breakdown strength. Furthermore, P-E loops of 10 wt% TiO
2-PEN were cycling measured at 150 °C for 10,000 times. The results show that this TiO
2-PEN hybrid exhibits excellent high-temperature dielectric stability as its
Ud and
η are maintained even after 10,000 cycling measurements at 150 °C (
f). Moreover, the dielectric properties of TiO
2-PEN hybrid were compared with those of PEN based composites reported in the literature (
Table S3) [
58,
59,
60,
61,
62]. TiO
2-PEN hybrid fabricated in this work not only shows the highest energy storage density but also demonstrates the highest
Tg, which suggests excellent dielectric properties at high temperatures.
. Dielectric constant (<strong>a</strong>) and loss (<strong>c</strong>) of 10 wt% TiO<sub>2</sub>-PEN at different temperatures; dielectric constant (<strong>b</strong>) and loss (<strong>d</strong>) of TiO<sub>2</sub>-PEN at 1 kHz with the variation of temperature; breakdown strength (<strong>e</strong>) and P-E loops (<strong>g</strong>) of TiO<sub>2</sub>-PEN at 150 °C; (<strong>h</strong>) energy density of 10 wt% TiO<sub>2</sub>-PEN at 25 and 150 °C; (<strong>i</strong>) <em>U</em><sub>d </sub>and <em>η</em> of TiO<sub>2</sub>-PEN at 150 °C; (<strong>f</strong>) <em>U</em><sub>d </sub>and <em>η</em> of 10 wt% TiO<sub>2</sub>-PEN at 150 °C during 10,000 times cycling measurement.
Methodology, Y.F.; Formal Analysis, S.B., Z.W., Y.L. and Y.J.; Writing—Original Draft Preparation, Y.F.; Writing—Review & Editing, R.W.; Funding Acquisition, L.W., X.H. and R.W.
Not applicable.
Not applicable.
Data can be provided upon request.
This research was funded by Natural Science Foundation of Shaanxi Province (2024-JC-YBQN-0140) and Key R&D Program Projects in Shaanxi Province (2023JBGS-22).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.