Found 4 results
Open Access
Review
07 August 2025Progress in the Study of Transition Metal-Based Carbon Nanotube Composites for Electrochemical Hydrogen Evolution
Hydrogen is an efficient, clean, and economical energy source, primarily due to its remarkably high energy density. Electrolytic water is considered an attractive and feasible method for hydrogen production. The high cost and scarcity of traditional Pt-based catalysts limit their large-scale application. Transition metals (TMs)-based composites, particularly those integrated with carbon nanotubes (CNTs), have emerged as promising alternatives due to their high conductivity, surface area, and ability to enhance the catalytic properties of TMs. Currently, there is no systematic summary of TMs-based CNTs composites for electrochemical hydrogen evolution reaction (HER). In this review, the main synthesis methods, including the wet chemical method, chemical vapor deposition, and electrochemical techniques, were first summarized. Then, the latest advancements of TMs/CNTs composites, focusing on their structure, electronic properties and superior HER catalytic performance, were systematically discussed. The catalytic mechanisms are meticulously examined, with particular emphasis on the pivotal role of CNTs in enhancing charge transfer and stabilizing metal nanoparticles. Finally, this review addresses the current challenges and future development directions for HER catalysts.

Open Access
Perspective
01 April 2025Perspectives on the Development in the Selective Oxidation of Glycerol to Value-Added Chemicals via Photoelectrocatalysis Coupled with Hydrogen Evolution
Harvesting sunlight to produce clean hydrogen fuel remains one of the main challenges for solving the energy crisis and ameliorating global warming. Photoelectrochemical (PEC) water splitting is considered to be a promising method for H2 production in the future. However, the efficiency still remains challenging due to the sluggish reaction dynamics for water oxidation. Recently, the thermodynamically favorable oxidation of glycerol in PEC systems has gained significant attention for its ability to produce value-added chemicals while simultaneously generating hydrogen. This process not only enhances the yield of high-value products but also minimizes energy consumption and reduces CO2 emissions. Valuable products from glycerol oxidation include 1,3-dihydroxyacetone (DHA), glyceraldehyde (GLD), tartronic acid (TA), formic acid (FA), and glyceric acid (GA). Thus, it is important to improve selectivity and productivity. In this work, we mainly summarize the recent research progress in improving the selectivity and productivity of glycerol upgrading products on the different photoanodes.
Open Access
Review
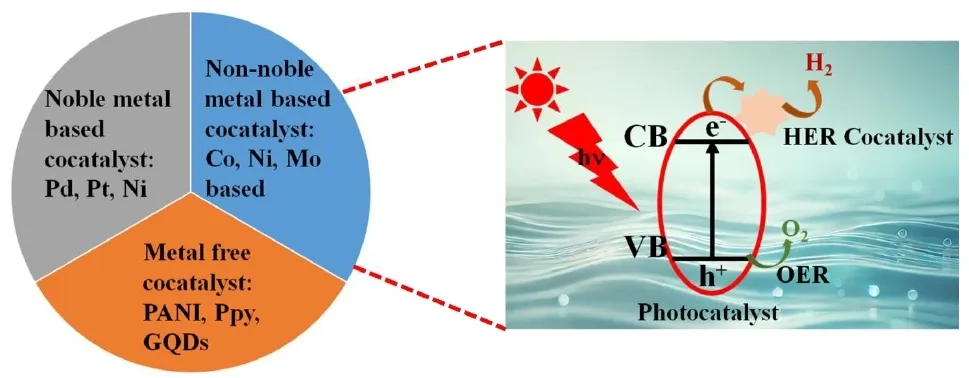
07 March 2025Unravelling the Role of Hydrogen Evolution Reaction Co-Catalysts in Photocatalytic Water Splitting: Mechanistic Insights and Material Strategies
The reliance on fossil fuels has led to a substantial increase in greenhouse gas emissions, presenting a critical environmental challenge. Addressing this issue necessitates the adoption of alternative renewable energy sources, with green hydrogen emerging as a promising candidate due to its high gravimetric energy density and absence of harmful emissions. Among the various hydrogen production techniques, photocatalytic technology has garnered significant attention for its dual potential to produce green hydrogen and degrade pollutants, thereby addressing both energy and climate crises. Efforts to scale photocatalytic technology for industrial applications have identified cocatalyst integration as a pivotal strategy, as it enhances reaction kinetics by lowering the activation energy and mitigating charge carrier recombination. This review comprehensively examines the hydrogen economy, the underlying principles of photocatalysis, recent technological advancements, key factors influencing photocatalytic reactions, the role of catalysts in hydrogen evolution reaction (HER) surface mechanisms, strategies for cocatalyst optimization, and future directions for the field.
Open Access
Article
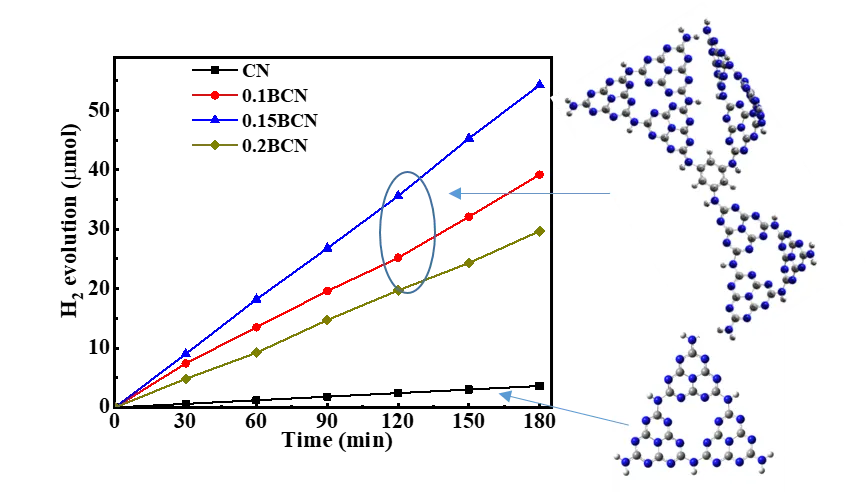
05 January 2024Benzene Bridged Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution
Turing the electronic structure by inserting certain functional groups in graphitic carbon nitride (g-C3N4, CN for short) skeleton through molecular doping is an effective way to improve its photocatalytic performance. Herein, we prepare a benzene bridged carbon nitride (BCN) by calcining urea and 1,3,5-tribromobenzene at elevated temperature. The introduction of benzene ring in g-C3N4 layers improves the separation efficiency and lifetime of photogenerated carriers, inhibits the recombination rate of electron/hole pairs, thus the performance of photocatalytic hydrogen evolution improves. The optimal hydrogen evolution rate of 1.5BCN reaches 1800 µmol/h·g, which is nine times that of the pure g-C3N4. DFT calculation proved the benzene bridged CN increased the distance of charge transfer (DCT) and the push-pull electronic effect of intramolecular electrons. This work may provide a pathway for preparing molecular doped g-C3N4 with improved photocatalytic performance.