Found 3 results
Open Access
Perspective
01 April 2025Perspectives on the Development in the Selective Oxidation of Glycerol to Value-Added Chemicals via Photoelectrocatalysis Coupled with Hydrogen Evolution
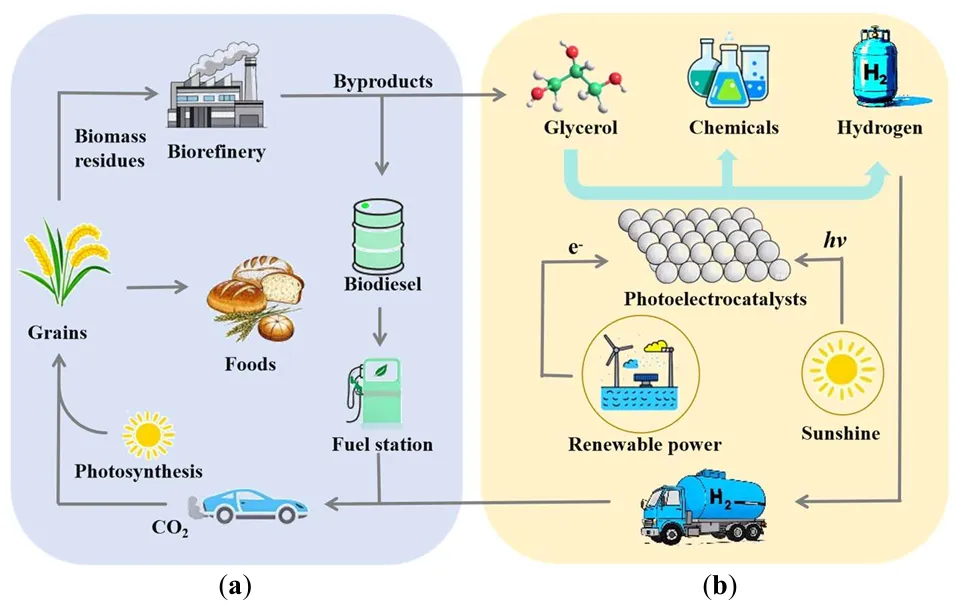
Harvesting sunlight to produce clean hydrogen fuel remains one of the main challenges for solving the energy crisis and ameliorating global warming. Photoelectrochemical (PEC) water splitting is considered to be a promising method for H2 production in the future. However, the efficiency still remains challenging due to the sluggish reaction dynamics for water oxidation. Recently, the thermodynamically favorable oxidation of glycerol in PEC systems has gained significant attention for its ability to produce value-added chemicals while simultaneously generating hydrogen. This process not only enhances the yield of high-value products but also minimizes energy consumption and reduces CO2 emissions. Valuable products from glycerol oxidation include 1,3-dihydroxyacetone (DHA), glyceraldehyde (GLD), tartronic acid (TA), formic acid (FA), and glyceric acid (GA). Thus, it is important to improve selectivity and productivity. In this work, we mainly summarize the recent research progress in improving the selectivity and productivity of glycerol upgrading products on the different photoanodes.
Open Access
Article
20 March 2025Metabolic Engineering and Genome-Wide Adaptive Evolution for Efficient Reduction of Glycerol in Industrial Saccharomyces cerevisiae
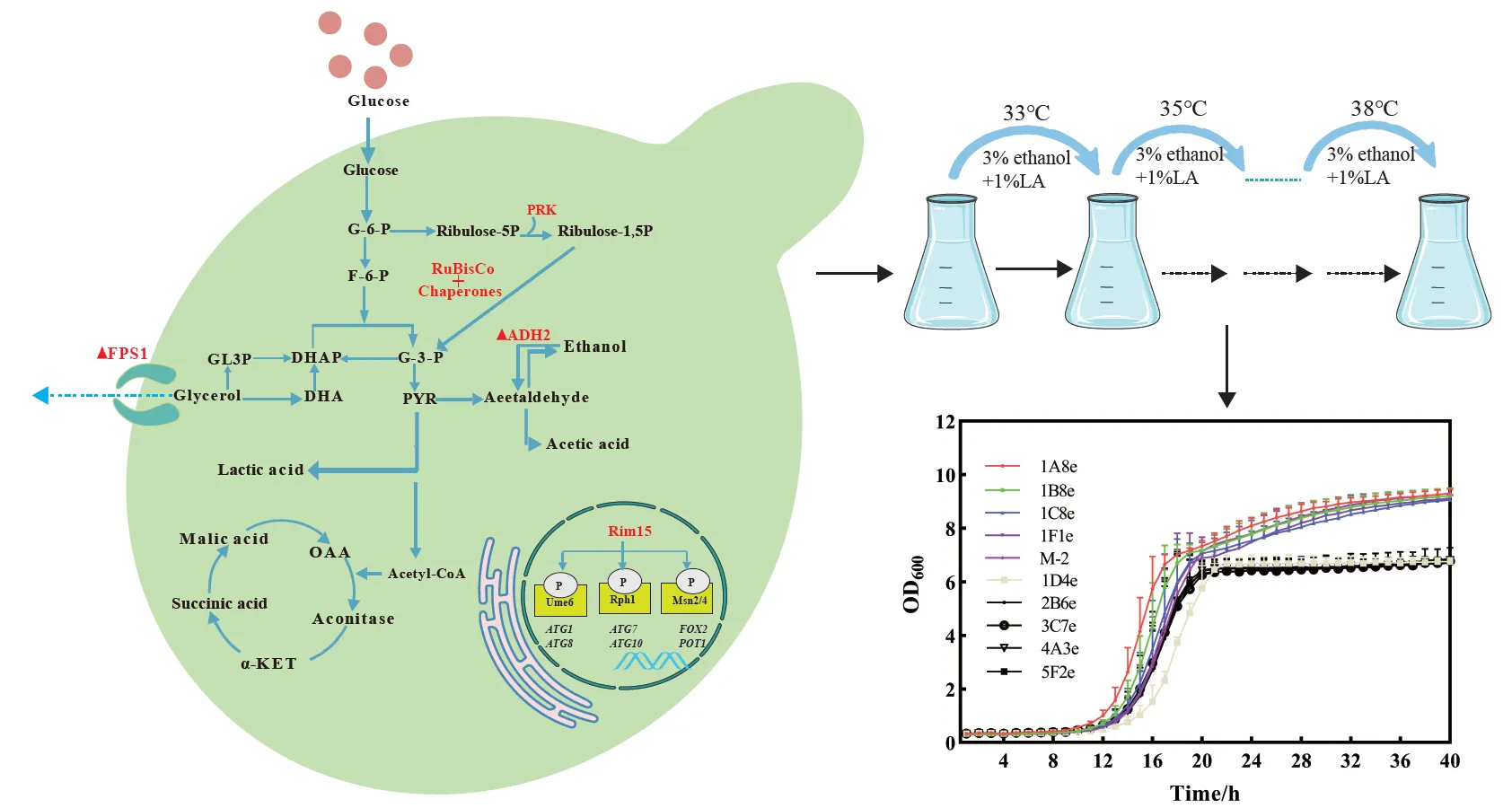
The production of glycerol as a major by-product during yeast-based bioethanol fermentation arises directly from the need to re-oxidize excess NADH, which reduces conversion efficiency. In this study, an optimized Cas9-based genome editing method was performed to develop a mixotrophic CO2-fixing industrial Saccharomyces cerevisiae by heterologous expression of ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO form Pseudomonas sp.) and phosphoribulokinase (PRK form Spinach). Additionally, the gene encoding alcohol dehydrogenase (ADH2) responsible for converting ethanol to acetaldehyde was deleted, while the great wall-family protein kinase Rim15 gene was overexpressed to facilitate the reduction in glycerol content. The resulting CO2-fixing yeast M-2 led to a 21.5% reduction of the by-product glycerol in corn mash fermentation cultures at 39 ℃. Moreover, we established a novel gene mutators mediated genome-wide mutations system that accumulates distinct mutations in the industrial S. cerevisiae strains under the stress conditions to improve the robustness in the S. cerevisiae strains efficiently.
Open Access
Article
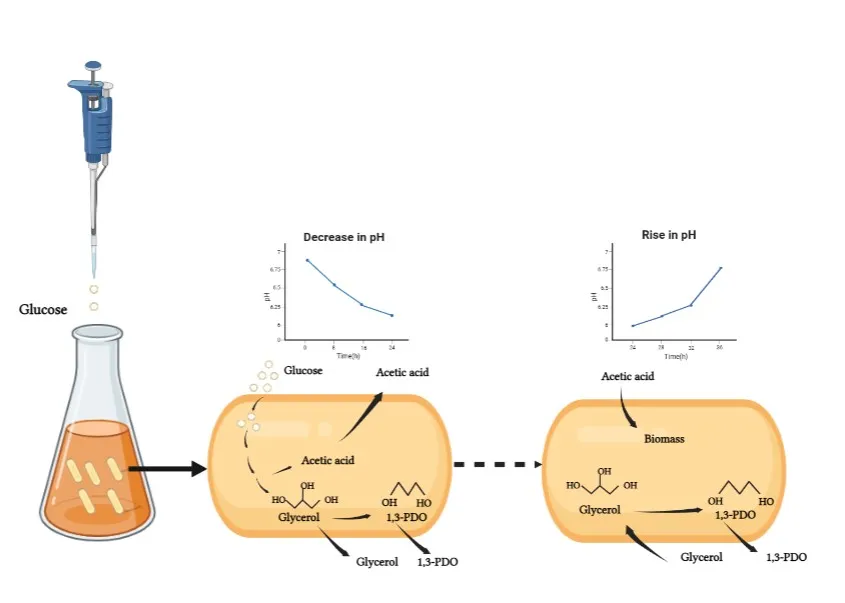
22 May 2023Fed-batch Self-regulated Fermentation of Glucose to Co-produce Glycerol and 1,3-propanediol by Recombinant Escherichia coli
As important bio-chemicals, glycerol and 1,3-propanediol (1,3-PDO) have been widely used in numerous fields, e.g., polymers, cosmetics, foods, lubricants, medicines, and so on. Bio-based 1,3-PDO is generally produced from glycerol or glucose by natural or recombinant strains, during which organic acids are always co-produced. In this work, acetic acid was also co-produced when 1,3-PDO was obtained from glucose by a recombinant strain of E. coli MG1655. Usually, a base was added to adjust the fermentation pH, resulting in the accumulation of organic salts and difficulty in the next down streaming process. Herein, a novel strategy was developed to consume the produced acetic acid by cells self in fed-batch self-regulated fermentation. The recombinant E. coli cells produced 48.33 g/L glycerol and 61.27 g/L 1,3-PDO with a total mass yield of 45.6% and without any other byproducts at the end of 5 fed-batch fermentations. The initial buffer pH, glucose concentration, pulse feeding sugar amount, time for a single batch fermentation and reducing acid were investigated by a series of comparative experiments. This fed-batch self-regulated fermentation has potential for the co-production of 1,3-PDO and glycerol, and will highlight the subsequent modification of recombinant E. coli strain by synthetic biology.