Found 2 results
Open Access
Article
19 July 2023Hydroxybenzoic Acid Production Using Metabolically Engineered Corynebacterium glutamicum
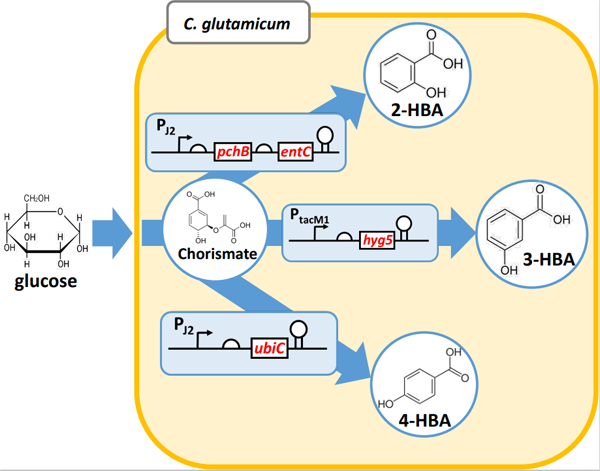
Hydroxybenzoic acids (HBAs), including 4-HBA, 3-HBA, and 2-HBA, are valuable platform chemicals for production of commodity materials and fine chemicals. Herein, we employed metabolic engineering techniques to enhance the production of these HBAs in Corynebacterium glutamicum ATCC 13032. Our approach augmented the shikimate pathway and eliminated genes associated with HBA degradation, particularly phenol 2-monooxygenase encoded by cg2966. Increased titers of 3-HBA and 4-HBA were achieved via selection of suitable promoters for 3-hydroxybenzoate synthase and chorismate pyruvate lyase. A tac-M1 promoter was suitable for chorismate pyruvate lyase expression and 8.3 g/L of 4-HBA production was achieved. Efficient production of 2-HBA was enabled by maintaining a balanced expression of isochorismate synthase and isochorismate pyruvate lyase. Consequently, strains KSD5-tacM1-H and KSD5-J2-PE exhibited production levels of 19.2 g/L of 3-HBA and 12.9 g/L of 2-HBA, respectively, using 1 L jar fermenter containing 80 g/L of glucose. Therefore, this engineered strain platform holds significant potential for production of other valuable products derived from chorismate.
Open Access
Article
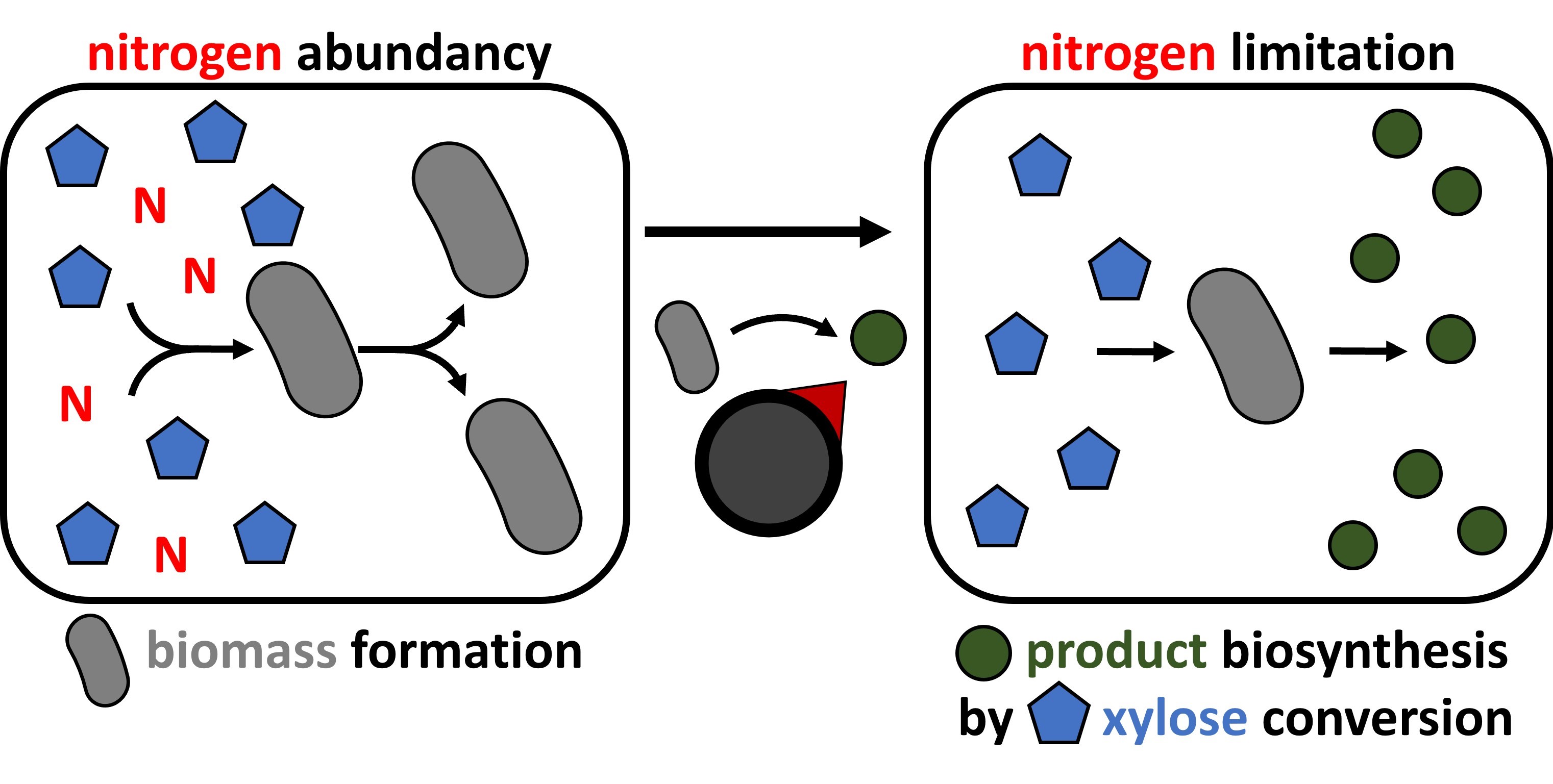
31 May 2023Nitrogen-controlled Valorization of Xylose-derived Compounds by Metabolically Engineered Corynebacterium glutamicum
The implementation of bioprocesses in an economically feasible and industrial competitive manner requires the optimal allocation of resources for a balanced distribution between biomass formation and product synthesis. The decoupling of growth and production in two-stage bioprocesses, aiming to ensure sufficient growth before the onset of production, is particularly relevant when target products inhibit growth. In order to avoid expensive inducer molecules, continuing process monitoring, elaborate individual process optimization, and strain engineering, we developed and applied nitrogen deprivation-induced expression of genes for product biosynthesis. Two native nitrogen deprivation-inducible promoters were identified and shown to function for dynamic growth-decoupled gene expression or CRISPRi-mediated gene knockdown in C. glutamicum with superior induction factors than the standard IPTG-inducible Ptrc promoter. Valorization of xylose to produce either the sugar acid xylonic acid or the sugar alcohol xylitol from xylose as sole source of carbon and energy was demonstrated. Competitive titers of up to 34 g·L−1 xylonate and 13 g·L−1 xylitol were achieved in two-stage processes. We discussed that the transfer to bioprocesses with C. glutamicum using carbon sources other than xylose appears straightforward in particular regarding production of growth-inhibitory compounds by their growth-decoupled fermentative production.