Found 3 results
Open Access
Review
04 September 2025Collagen Biosynthesis to Engineered Biomaterials: Molecular Design, Synthetic Strategy, and Biomedical Application
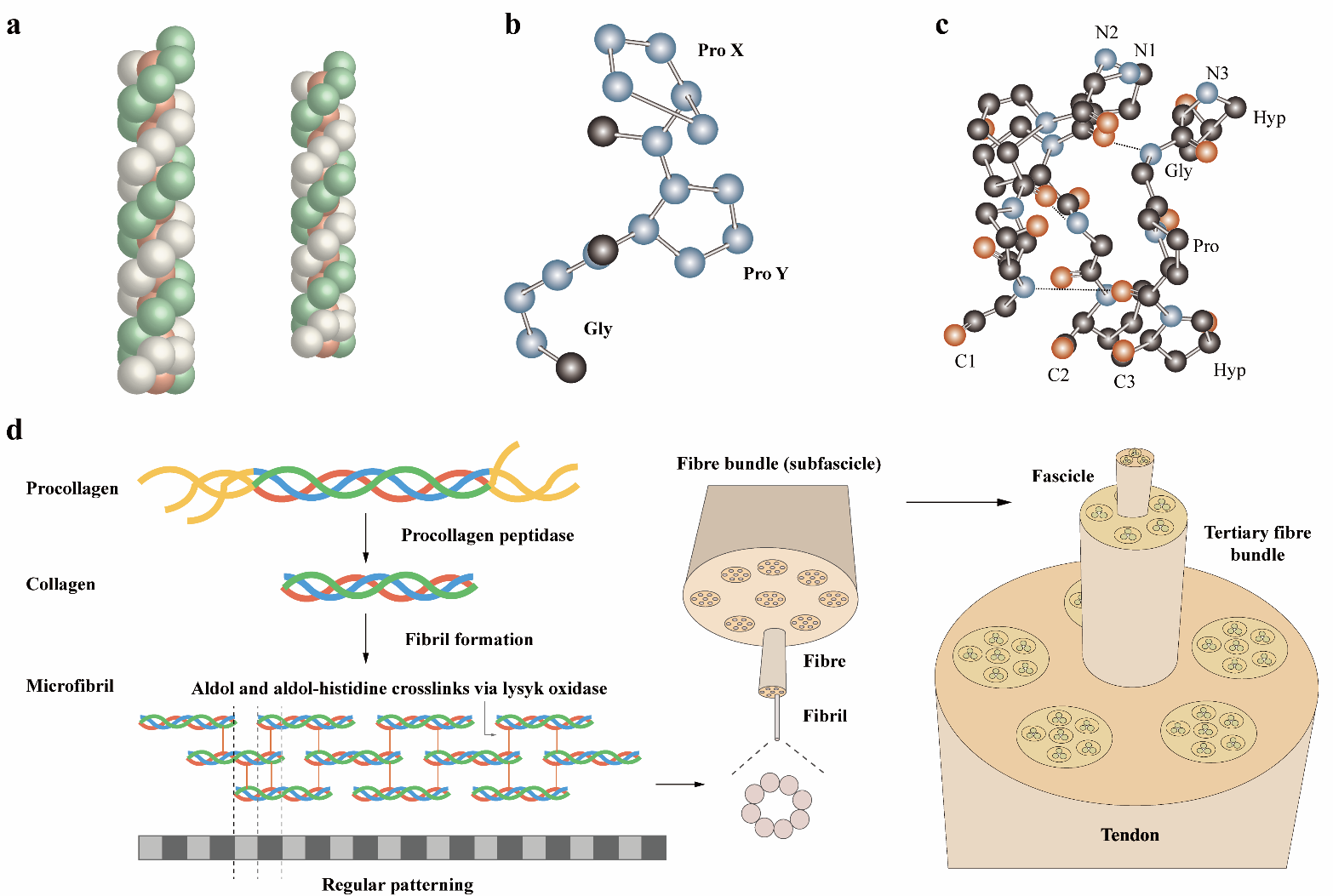
Collagen, a principal component of the extracellular matrix, provides mechanical strength and stability to tissues and organs through its structural organization. Its biocompatibility has established it as a crucial material in biomedical applications such as drug delivery systems, cell culture matrices, and tissue engineering scaffolds. However, the use of animal-derived collagen carries risks of pathogen transmission, which has driven research towards developing synthetic collagen alternatives. Advances in AI-assisted protein engineering are accelerating the design of synthetic collagens and their applications in biomaterials. This review examines collagen’s structural characteristics, biosynthesis strategies, biological activities as well as AI-assisting engineering.
Open Access
Article
01 August 2025Eastern Lubber Grasshopper Extract-Inspired Silver Nanoparticles Selectively Inhibit Methicillin-Resistant Staphylococcus aureus
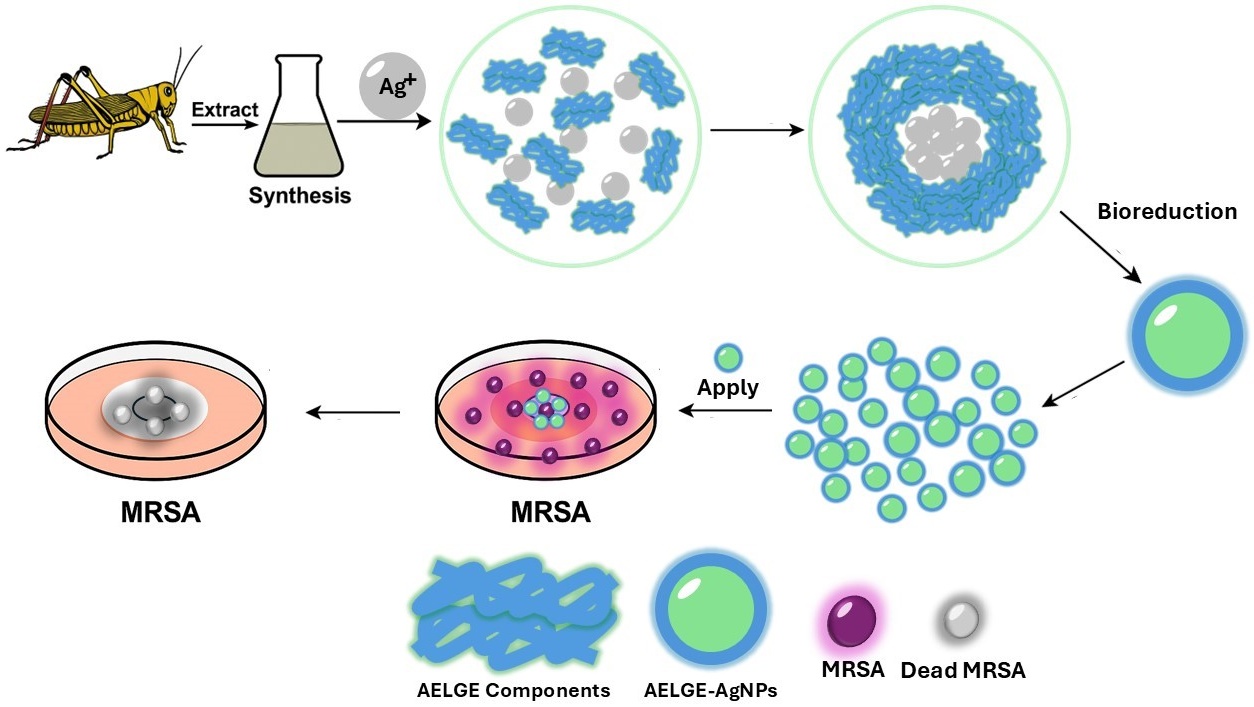
Silver nanoparticles (AgNPs) were synthesized using a protein/polypeptide-rich aqueous extract from the Eastern lubber grasshopper (Romalea microptera), as a natural reducing and capping agent. The resulting AgNPs exhibited relatively uniform sizes (10–60 nm) and were characterized by Fourier Transform Infrared Spectroscopy (FTIR), Ultraviolet-visible (UV-Vis) spectroscopy, Transmission electron microscopy (TEM), and Scanning Electron Microscopy (SEM). Disk diffusion tests against five bacterial strains (Methicillin-resistant Staphylococcus aureus (MRSA), Burkholderia cenocepacia, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli) demonstrated that the insect-extract-induced AgNPs selectively and significantly inhibited MRSA growth, with an average value of zone of inhibition of 9.16 ± 1.11 mm (n = 4). Statistical analysis confirmed the superior antibacterial activity of the Eastern lubber grasshopper-derived AgNPs against MRSA compared to citrate-capped AgNPs and free silver ions. These findings reveal the potential of insect-derived AgNPs as selective, green-synthesized antibacterial agents with enhanced efficacy and reduced side effects, particularly against antibiotic-resistant pathogens.
Open Access
Review
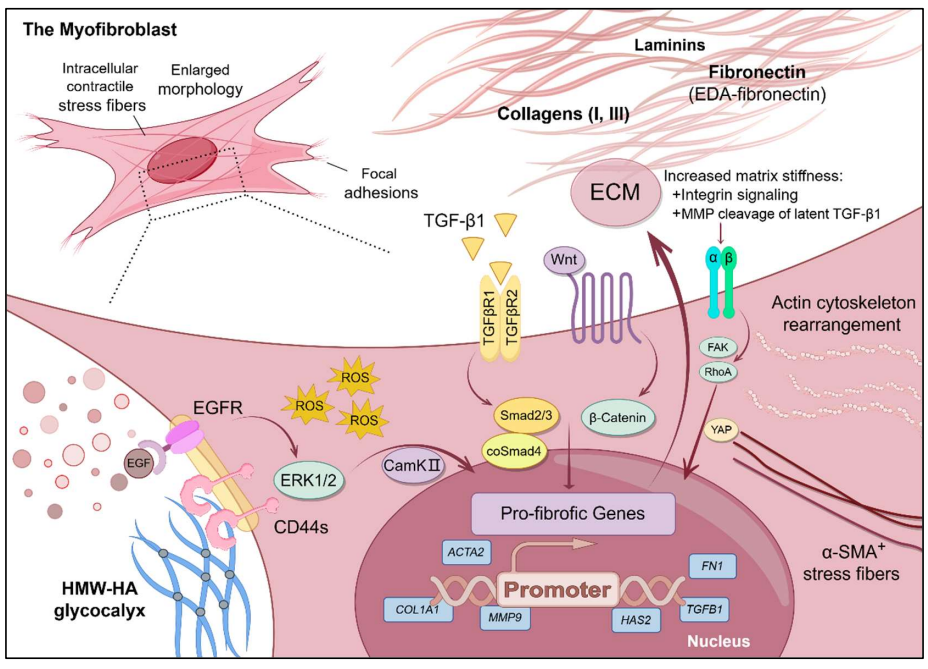
22 November 2024Dermal Fibrosis and the Current Scope of Hydrogel Strategies for Scarless Wound Healing
Dermal fibrosis poses a significant challenge in wound healing, affecting both the appearance and functionality of the scarred skin tissue. Beyond aesthetic implications, fibrosis can lead to pruritus, chronic pain, loss of mechanical flexibility, and impeded restoration of skin appendages, blood vessels, and nerves. Therefore, scar prevention remains a priority in wound management, and hydrogels, with their hydrophilic three-dimensional network and extracellular matrix-mimicking properties, have emerged as promising biomaterials for achieving scarless wound regeneration. In this review, we explore advancements in various hydrogel strategies designed to regulate myofibroblast differentiation, control the wound microenvironment, and mitigate dermal fibrosis. We provide an overview of dermal fibrosis, the scar-forming cells involved, and the various types of dermal scars. We then summarise advancements made in antifibrotic hydrogel formulations, emphasizing their practical applications in scarless skin wound healing. By reviewing the current research landscape and highlighting key hydrogel-based biomaterial strategies employed in this field, we aim to offer insights into design principles and underlying mechanisms of action. We intend for this review to serve as a valuable resource for researchers and clinicians interested in entering this field or exploring the potential of hydrogels to promote scarless wound healing.