Synergistic Natural Products in Anti-Ageing: Mechanistic Insights, Experimental Evidence, and Translational Perspectives
Received: 18 November 2025 Revised: 13 January 2026 Accepted: 20 January 2026 Published: 26 January 2026
© 2026 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
1.1. Background for Ageing and Anti-Ageing
Ageing represents a progressive decline in physiological integrity driven by the cumulative effects of molecular and cellular damage over time [1,2]. In contrast, anti-ageing refers to scientific and clinical strategies designed to delay this process, prevent age-related diseases, and preserve physical, functional, and aesthetic health [3,4].
Natural compounds are increasingly recognised as pivotal components of anti-ageing strategies, as they exhibit antioxidant, anti-inflammatory, photoprotective, antimicrobial, wound-healing, and DNA-repair activities [5]. These bioactive molecules, derived from terrestrial and marine organisms, fungi, and microorganisms, include secondary metabolites such as flavonoids, phenolic acids, polysaccharides, and lipopeptides that exert measurable anti-ageing effects through multiple biological mechanisms [6,7,8,9,10,11].
1.2. Literature Search Strategy
Publications on the anti-ageing effects of natural products were identified through a comprehensive two-stage search conducted across the Web of Science Core Collection, Google Scholar, and the University of Auckland Libraries (2005–2025). The initial scoping stage, using the keywords “anti-ageing”, “natural products”, and “functional food”, was performed to outline overall research trends and thematic distributions. A subsequent targeted search combined “anti-ageing” with polyphenols, terpenoids, polyamines, polysaccharides, fatty acids, bioactive peptides, and multi-component natural products to identify mechanistic and experimental studies. Grey literature and reference verification were included through Google Scholar and institutional resources, while studies restricted to topical or cosmetic formulations were excluded. Additional relevant references were identified through citation tracking.
Keyword co-occurrence and timeline analyses were conducted using CiteSpace (v6.2.R4) to visualise research dynamics within the dataset. The results revealed a progressive shift from early studies focused on general biological processes, such as “mammalian ageing” and “vitamin C”, to more recent research emphasising mechanistic and multi-component strategies involving natural bioactives and dietary interventions (Figure 1). This trend highlights the increasing integration of molecular biology, nutrition, and pharmacognosy in anti-ageing research.
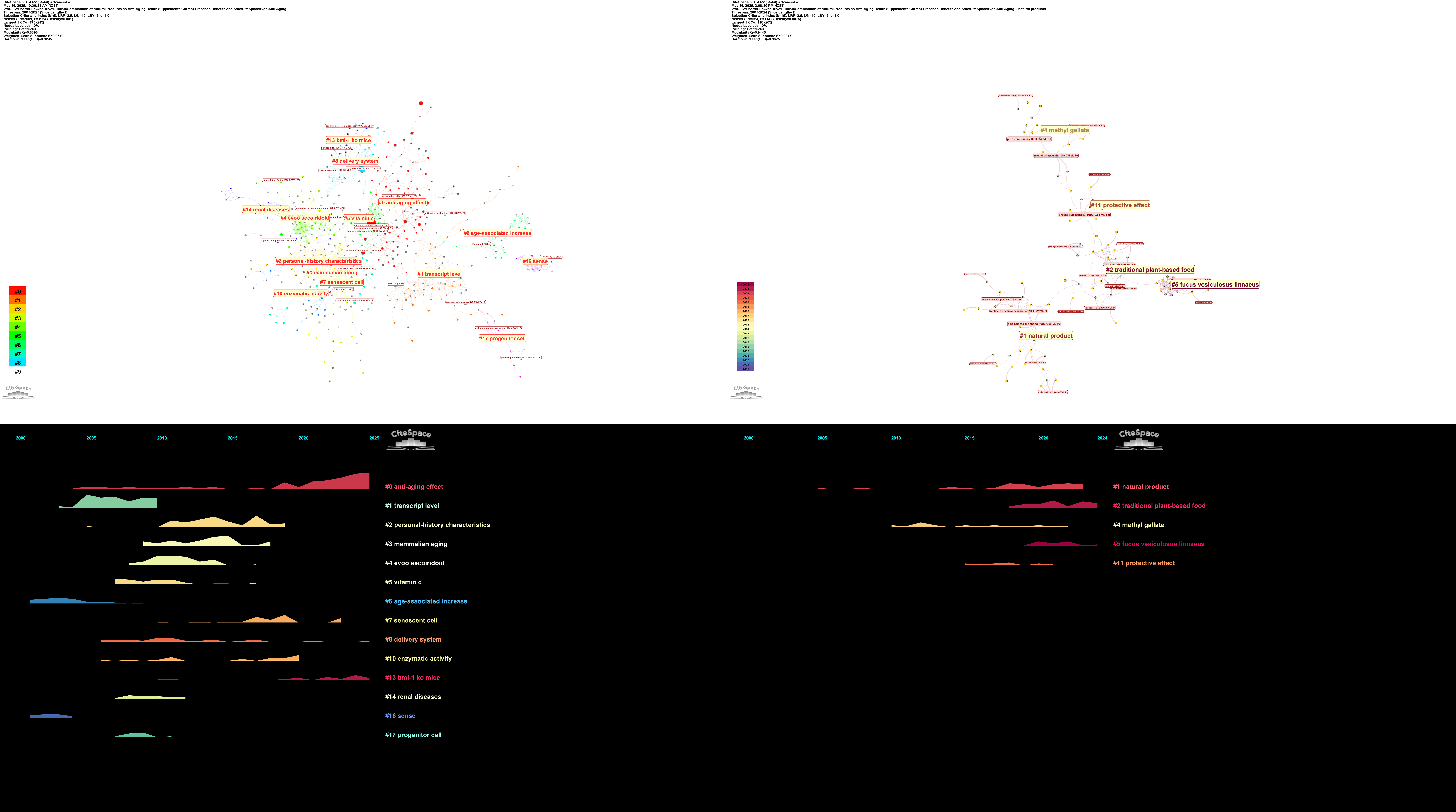
Figure 1. Keyword co-occurrence and timeline mapping of anti-ageing research on natural products (2005–2025). Notes: Upper panels show clustered keyword networks, and lower panels display their temporal evolution. Colours indicate cluster identity and chronological order. Analysis conducted in CiteSpace (v6.2.R4) using data from the Web of Science Core Collection.
1.3. Aim of This Review
This review synthesises current evidence on the anti-ageing potential of major classes of natural bioactives, including polyphenols, terpenoids, polyamines, polysaccharides, fatty acids, and bioactive peptides. It focuses on their roles in modulating oxidative stress, inflammation, mitochondrial function, and cellular senescence, and evaluates naturally derived multi-component products to elucidate how complex compositions contribute to anti-ageing effects. By integrating findings from both single-compound and combined formulations, this review proposes mechanistic hypotheses for synergistic interactions among natural products in mitigating age-related decline.
2. Biological Mechanisms of Ageing and Anti-Ageing Strategies
2.1. Theory of Ageing Causes
Ageing is characterised by a progressive loss of physiological integrity that impairs function, increases vulnerability to stress and disease, and ultimately culminates in organismal mortality [12]. Numerous hypotheses have been proposed to explain age-related changes; however, they often conflict with one another, and no single model can fully account for the complexity of the ageing process [13].
Recent research has introduced novel perspectives, such as the five-factor theory proposed by Obradovic [14], which conceptualises ageing as a multifactorial process driven by structural alterations in cells, the cessation of cell division, and impaired stem-cell signalling that culminates in systemic functional decline. While this framework integrates both cellular and evolutionary perspectives, it remains limited in identifying the precise molecular drivers of these structural changes and in defining the conditions under which stem cells may be reactivated in long-lived species. These acknowledged gaps underscore the importance of situating this model within a broader theoretical context.
Although this theory presents an innovative perspective, it remains limited in several respects. Specifically, it does not clearly define the molecular or environmental factors underlying these structural alterations, nor does it adequately explain the conditions under which stem cells may be reactivated to delay ageing, as observed in certain long-lived species. These limitations, consistent with gaps acknowledged by the original author, underscore the need to situate this theory within the broader context of ageing research.
Contemporary biological theories are broadly categorised into programmed theories and damage- or error-based theories. Programmed theories propose that ageing follows a genetically regulated biological timetable, wherein altered gene expression, hormonal regulation, and immune decline collectively drive physiological deterioration [2,13,15,16]. The programmed longevity theory views ageing as a continuation of developmental processes governed by specific genes. The endocrine theory links age-related hormonal shifts to the regulation of biological clocks. The immunological theory attributes senescence to genetically programmed decline in immune function [17,18] (Table 1).
Table 1. Classical ageing theories and their mechanistic pathways.
|
Theory Category |
Representative Models |
Description |
Key Mechanistic Features/Targets |
|---|---|---|---|
|
Programmed Theories |
Programmed Longevity |
Ageing is considered a continuation of development regulated by a genetic timetable, resulting from the sequential switching on and off of specific genes. |
|
|
Endocrine Theory |
Biological clocks regulate the pace of ageing through hormones. Hormonal signalling pathways control metabolism, growth, and repair, thereby influencing lifespan. |
|
|
|
Immunological Theory |
The immune system is genetically programmed to decline with age, leading to increased susceptibility to infections, chronic inflammation, and ageing-related diseases. |
|
|
|
Damage/Error Theories |
Wear-and-Tear Theory |
Ageing results from prolonged functional stress that gradually damages and degrades cells and tissues, leading to organ dysfunction and death. |
|
|
Rate-of-Living Theory |
Higher metabolic rates correlate with shorter lifespans. The theory suggests that organisms with higher energy expenditure age faster and die sooner. |
|
|
|
Cross-Linking Theory |
Accumulation of cross-linked proteins impairs cellular and tissue functions. |
|
|
|
Free Radical Theory |
Ageing results from the accumulation of cellular damage caused by ROS. |
|
|
|
Mitochondrial DNA Damage Theory |
Ageing is driven by mitochondrial dysfunction and progressive mtDNA damage, which amplify oxidative stress and trigger cell death. |
|
This table summarises the major classical theories of ageing, categorised into programmed and damage/error models. Each theory is briefly described, and its key mechanistic features and molecular targets are outlined to highlight the distinct biological processes implicated in ageing. These mechanistic insights provide a conceptual framework for comparing traditional hypotheses with emerging perspectives and for guiding future research on anti-ageing interventions [2,13,15,16,17,18,19,20,21,22,23].
In contrast, damage- or error-based theories attribute ageing to the cumulative effects of environmental and metabolic stressors rather than to predetermined genetic programming. These frameworks encompass the wear-and-tear, rate-of-living, cross-linking, free radical, and mitochondrial DNA damage theories. The wear-and-tear theory posits that prolonged functional stress leads to cellular and tissue degradation, whereas the rate-of-living theory associates higher metabolic rates with reduced lifespan [2,13,15]. The cross-linking and free radical theories emphasise molecular damage arising from accumulated protein cross-links and reactive oxygen species (ROS) [19,21,22]. The mitochondrial DNA damage theory further links mitochondrial dysfunction to oxidative stress and cell death [18] (Table 1).
Collectively, these frameworks offer complementary perspectives on the intrinsic and extrinsic drivers of ageing and form a conceptual foundation for anti-ageing research. While each model accounts for only part of the process, together they highlight the multifactorial nature of biological ageing and the intricate interplay among genetic regulation, metabolic homeostasis, and repair mechanisms of cellular damage.
2.2. Theory of Anti-Ageing
The interplay among ageing triggers, phenotypic traits, and age-related diseases, whether genetically inherited or acquired, has attracted growing scientific attention in recent decades. Concurrent advances in biotechnology and biomedical sciences have expanded both the design and the implementation of anti-ageing interventions. Contemporary theories propose that phenotypic ageing arises from dynamic interactions between intrinsic genetic profiles and modifiable environmental factors, including diet, physical activity, and other environmental exposures (Equation (1)). This conceptual model illustrates the interdependence between genetic determinants and environmental factors in shaping the ageing phenotype [24].
Equation (1). Phenotypic Determinants of Ageing
|
```latex\begin{aligned} & Phenotypic\ Ageing \\ & = Genotypes \\ & + External\ Factors\ (e.g.,\ diet,\ lifestyle\ and\ environmental\ conditions) \end{aligned}``` |
(1) |
Current anti-ageing strategies are generally classified into three complementary frameworks: geroprotection, which aims to prevent or delay damage accumulation; rejuvenation, which focuses on restoring physiological function; and regeneration, which promotes the repair or replacement of aged or damaged tissues [25,26].
Building upon these foundational principles, a wide range of interventions has emerged, reflecting advances in mechanistic understanding of ageing. These approaches can be broadly categorised into five domains: lifestyle interventions, microbiome modulation, genetic and regenerative strategies, molecular targeting, and emerging technologies [25,27].
Lifestyle-based strategies, including caloric restriction, antioxidant-rich diets, regular physical activity, and adequate sleep, help maintain metabolic balance, reduce oxidative stress, and enhance autophagic turnover, thereby supporting circadian synchrony and systemic homeostasis [24,25]. Molecular and regenerative approaches encompass several interrelated mechanisms, such as nicotinamide adenine dinucleotide (NAD+) metabolism, senescence clearance, mitochondrial biogenesis, and the use of DNA-methylation clocks to evaluate biological ageing [20,23,26,27].
Finally, emerging technologies, including epigenetic remodelling, synthetic biology, and biomarker-guided feedback systems, represent the frontier of precision ageing medicine [26] (Table 2).
Table 2. Overview of Principal Anti-Ageing Strategies and Their Biological Foundations.
|
Strategy Category |
Representative Examples |
Description |
Core Biological Focus |
|---|---|---|---|
|
Lifestyle Interventions |
Caloric restriction, intermittent fasting, antioxidant-rich diet, exercise, and sleep optimisation |
Lifestyle-based approaches support systemic homeostasis by reducing oxidative and metabolic stress, enhancing autophagy, and promoting cellular repair. These strategies influence multiple hallmarks of ageing through metabolic and circadian regulation. |
Redox balance • Energy metabolism • Circadian synchrony • Autophagic turnover |
|
Microbiome Modulation |
Probiotics, prebiotics, high-fibre diet |
Improves microbial diversity and intestinal barrier integrity, reducing systemic inflammation and supporting immune–metabolic communication. |
Gut–immune axis • Inflammation control • Metabolic resilience |
|
Genetic and Regenerative Approaches |
Telomere maintenance (hTERT activation), stem cell therapy, cellular reprogramming |
Targets chromosomal stability and cellular renewal to delay senescence and restore tissue function. |
DNA repair • Epigenetic stability • Stem-cell regeneration |
|
Molecular Targeting |
NAD+ supplementation, senolytics, mTOR modulators |
Modulates signalling pathways associated with cellular stress, autophagy, and senescence to maintain metabolic balance. |
Energy sensing • Proteostasis • Cellular senescence control |
|
Emerging Technologies |
Epigenetic remodelling, synthetic biology tools, biomarker-guided interventions |
Employ advanced biotechnologies for precision monitoring and reprogramming of ageing processes, mostly at the pre-clinical stage. |
Epigenetic reversal • Digital biomarker feedback • System-level precision modulation |
This table categorises contemporary anti-ageing interventions into five major domains: lifestyle interventions, microbiome modulation, genetic and regenerative approaches, molecular targeting, and emerging technologies. For each category, representative examples are provided along with a concise description and their principal mechanistic features, illustrating how each strategy engages specific biological processes implicated in ageing and age-related functional decline [24,25,26,27].
2.3. Molecular Pathways Underlying Ageing and Anti-Ageing Regulation
Ageing arises from a network of interconnected signalling cascades that coordinate metabolism, redox balance, inflammation, autophagy, and cellular renewal. These cascades constitute the molecular foundation of anti-ageing research and represent key targets for nutritional and pharmacological interventions. They act as nodal regulators linking nutrient sensing, energy metabolism, and stress responses, integrating physiological resilience with longevity outcomes [28,29,30,31,32]. Collectively, these signalling cascades form a multilayered regulatory network that integrates metabolic, redox, inflammatory, and immune regulation to modulate ageing (Figure 2) [33,34,35].
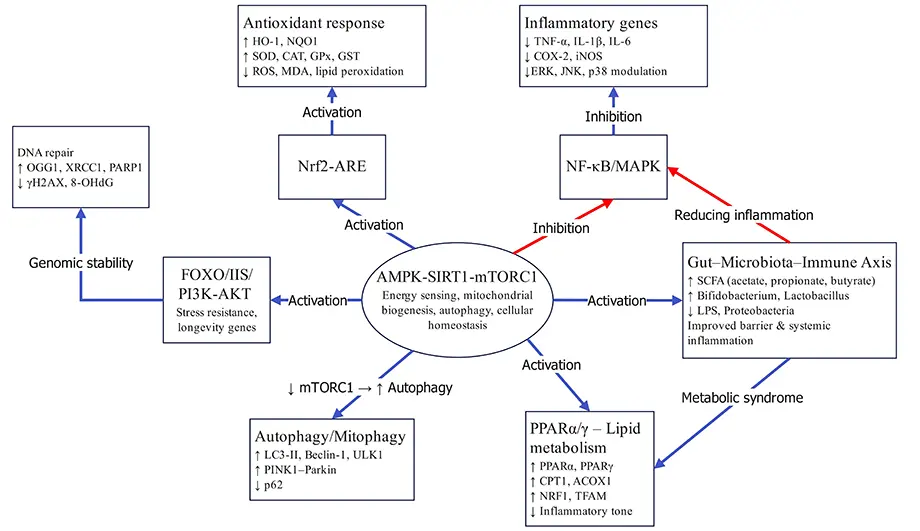
Figure 2. Integrated molecular network underlying anti-ageing regulation by natural products. Notes: Natural products activate the AMPK–SIRT1–mTORC1 axis and its downstream antioxidant, anti-inflammatory, metabolic, autophagic, and microbiota-related pathways, thereby enhancing mitochondrial function, genomic stability, and systemic homeostasis.
2.3.1. AMPK-SIRT1-mTORC1 Axis
The AMP-activated protein kinase (AMPK)–sirtuin 1 (SIRT1)–mechanistic target of rapamycin complex 1 (mTORC1) axis acts as a central metabolic switch that controls energy sensing, mitochondrial biogenesis, and autophagy. Activation of AMPK and SIRT1 enhances catabolic efficiency and cellular stress tolerance through fatty acid oxidation and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α)–mediated mitochondrial function, whereas inhibition of mTOR limits anabolic overactivation and suppresses cellular senescence [36,37,38]. Convergent evidence from multiple classes of natural products indicates that modulation of this axis improves mitochondrial quality control, attenuates growth factor signalling, and supports chromatin stability [28,30,39].
2.3.2. Nrf2-ARE Pathway
The nuclear factor erythroid 2–related factor 2 (Nrf2)–antioxidant response element (ARE) pathway regulates cellular redox homeostasis. Under oxidative stress, Nrf2 translocates to the nucleus and induces the expression of detoxification and antioxidant genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), and superoxide dismutase (SOD). Sustained activation mitigates ROS accumulation, prevents lipid peroxidation, and protects macromolecules from oxidative damage [40,41,42]. Studies on polyphenols, terpenoids, and polysaccharides have shown that these compounds enhance Nrf2 nuclear localisation and downstream gene transcription, thereby improving antioxidant capacity and delaying functional decline [33,43,44].
2.3.3. NF-κB and MAPK Cascades
The nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) cascades mediate inflammatory and stress-related transcriptional responses. NF-κB activation upregulates pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), and cyclooxygenase-2 (COX-2), whereas MAPK members, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38), regulate cellular adaptation to oxidative and metabolic stress. Persistent overactivation of these pathways contributes to inflammageing and tissue degeneration [45,46]. Natural products such as polyphenols, terpenoids, peptides, and unsaturated fatty acids have been shown to inhibit NF-κB nuclear translocation and modulate MAPK phosphorylation, thereby reducing chronic low-grade inflammation and preserving tissue integrity [28,37,38,39].
2.3.4. FOXO/IIS/PI3K-AKT Signalling Pathway
Forkhead box O (FOXO) transcription factors act downstream of the insulin/IGF-1 signalling (IIS) and phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT) cascades. Reduced IIS or AKT activity promotes FOXO nuclear translocation and the activation of genes related to antioxidant defence, DNA repair, and cellular maintenance, linking nutrient signalling with metabolic rate and lifespan regulation across multiple species [47,48,49]. Evidence from polysaccharides, peptides, and terpenoids demonstrates that these compounds can rebalance IIS–AKT activity, enhance FOXO-dependent transcription, and thereby improve stress resistance and longevity phenotypes [28,50,51].
2.3.5. PPAR and Lipid-Metabolism Axis
Peroxisome proliferator-activated receptors (PPARs) regulate lipid utilisation, adipogenesis, and inflammatory resolution. Activation of peroxisome proliferator-activated receptor α (PPARα) and peroxisome proliferator-activated receptor γ (PPARγ) enhances fatty acid oxidation, improves insulin sensitivity, and suppresses systemic inflammation, whereas dysregulated PPAR signalling contributes to metabolic ageing and redox imbalance [52,53,54]. Bioactive fatty acids and terpenoids frequently act as PPAR ligands, promoting mitochondrial biogenesis and metabolic flexibility while reducing lipid-induced stress in ageing tissues [55,56,57].
2.3.6. Gut-Microbiota-Immune Axis
The gut–microbiota–immune axis has emerged as a systemic determinant of ageing. Balanced microbial communities support nutrient absorption, short-chain fatty acid production, and immune tolerance, whereas dysbiosis promotes chronic inflammation and metabolic dysfunction. Natural products such as polyphenols, polysaccharides, fatty acids, and polyamines reshape microbial composition and metabolite profiles, reinforce mucosal barrier integrity, and mitigate age-related immune dysregulation [58,59,60,61].
3. Experimental and Clinical Evidence of Natural Products in Anti-Ageing
3.1. Experimental and Clinical Evidence of Individual Natural Products
3.1.1. Current Evidence of Polyphenols for Anti-Ageing
Polyphenols from diverse dietary and botanical sources exhibit measurable anti-ageing effects across cellular, organismal, and clinical systems. Building on earlier mechanistic insights, current evidence confirms their capacity to modulate lifespan, redox balance, and health-span through antioxidant and metabolic regulation (Table 3) [62,63,64,65].
Table 3. Summary of experimental evidence on polyphenols exhibiting anti-ageing effects across models.
|
Source/Material |
Principal Polyphenols |
Experimental Models |
Main Anti-Ageing Outcomes |
References |
|---|---|---|---|---|
|
Green and black tea (Camellia sinensis) |
EGCG, theaflavins, catechins |
C. elegans, yeast, mammalian cells |
↑ SOD, CAT (20–35%); ↓ lipid peroxidation (20–40%); activated Nrf2–ARE and AMPK–SIRT1 pathways; improved mitochondrial function and stress resistance. |
|
|
Pomegranate (Punica granatum) |
Ellagitannins, punicalagins |
Human fibroblasts, mouse |
Upregulated HO-1 and NQO1; ↓ ROS and protein carbonyls; strengthened collagen stability and redox balance via Nrf2–SIRT1 regulation. |
|
|
Indian gooseberry (P. emblica) |
Emblicanin A/B, gallic acid, ellagic acid |
C. elegans, rodent cells |
Activated Nrf2–ARE and SIRT1; ↑ SOD/CAT, ↓ MDA (~30%); delayed senescence and extended lifespan (~15%). |
|
|
Peony bark and stamen (Paeonia suffruticosa) |
Paeoniflorin, paeonol, catechins |
C. elegans, neuronal cells |
Inhibited NF-κB and MAPK; ↓ pro-inflammatory cytokines; enhanced neuronal antioxidant defence and stress tolerance. |
|
|
Fermented polyphenol products (e.g., mulberry, tea co-ferments) |
Catechins, phenolic acids (biotransformed forms) |
C. elegans, mouse, microbial assays |
↑ Bifidobacterium and Lactobacillus; ↑ SCFAs synthesis; ↓ systemic inflammation and improved metabolic homeostasis. |
|
|
Olive (Olea europaea) |
Hydroxytyrosol, tyrosol |
Yeast, mammalian cells |
↓ ROS generation, promoted mitochondrial biogenesis and autophagy, improved cellular energy metabolism. |
[74] |
|
Chamomile (Matricaria chamomilla) |
Lignisulide, ferulic acid |
C. elegans, neuronal cells |
Activated FOXO; enhanced neuronal regeneration and antioxidant defence; preserved synaptic and mitochondrial integrity. |
|
|
Fermented red ginseng extracts |
Ginsenoside-linked phenolics |
Mouse, microbial models |
↑ SOD and CAT; restored gut microbial balance; ↓ oxidative stress and inflammation; improved metabolic function. |
[77] |
Quantitative values (↑ increase; ↓ decrease) are derived from the referenced studies, encompassing C. elegans, yeast, rodent, human fibroblast, and microbial fermentation models.
Tea-derived polyphenols show some of the most consistent results. Green-tea catechins, including epigallocatechin gallate (EGCG), epicatechin gallate (ECG), and epigallocatechin (EGC), extended C. elegans lifespan by 12–24% and increased antioxidant-enzyme activity by about 1.6-fold, while improving locomotor function and resistance to oxidative stress by 30–40% [62,63,64,65]. Processing methods such as fermentation or roasting further increased total polyphenol content and free-radical-scavenging capacity [74,78,79].
Fruit-derived polyphenols also show robust antioxidant and lifespan-supporting effects. Pomegranate ellagitannins increased fibroblast viability by 25% and reduced intracellular ROS by nearly 50% [66,67]. Rose-petal polyphenols reduced protein carbonylation by over 30% and increased collagen-related gene expression, indicating improvements in dermal-ageing phenotypes [80,81,82]. Phyllanthus emblica (P. emblica) polyphenols extended C. elegans lifespan by 18.5% and increased SOD and CAT activities while reducing lipid peroxidation by about 36% [68,69,83]. Kiwifruit extracts further improved antioxidant capacity and survival across ageing models [77,84,85].
Polyphenol-rich medicinal herbs provide additional anti-ageing support. L. chuanxiong extracts enriched in ligustilide and ferulic acid extended lifespan by about 16% and reduced ROS accumulation, while improving mitochondrial integrity and neuronal regeneration [75,76,86,87,88]. Peony-bark phenolics, including paeoniflorin and catechin derivatives, improved nematode stress tolerance by 40% and decreased lipid peroxides by about 60% [51,70,71]. Hydroxytyrosol from olive showed enhanced antioxidant activity and chronological-lifespan extension in cellular and yeast models [74], while ginger polyphenols exhibited similar antioxidant efficacy [80].
Enhancement strategies, such as fermentation, further increase bioactivity. Fermented mulberry and Siraitia grosvenorii polyphenols produced 1.9–2.3-fold increases in antioxidant indices and extended C. elegans lifespan by 18–22%, concurrent with elevated levels of quercetin, gallic acid, and other phenolic acids [73,89]. Co-fermentation further increased SOD and silent information regulator 2 (SIR2) expression and improved gut-microbial profiles [77,90]. Polyphenol–polysaccharide complexes and synergistic formulations containing catechins, procyanidins, and phenolic acids increased SOD and CAT activity by 50–65%, accompanied by parallel gains in antioxidant capacity [72,77,91,92].
Taken together, current evidence shows that polyphenols mitigate oxidative and metabolic hallmarks of ageing, promote stress resilience, and support overall health-span across diverse biological systems.
3.1.2. Current Evidence of Terpenoids for Anti-Ageing
Terpenoids are widely distributed in medicinal plants and functional foods, and an increasing body of experimental evidence demonstrates their quantifiable anti-ageing efficacy. Improvements in redox status, inhibition of extracellular-matrix-degrading enzymes, and enhanced cellular vitality have been consistently observed across major subclasses, particularly sesquiterpenes, diterpenes, and triterpenoids derived from botanicals such as ginger, frankincense, peony, propolis, and coffee [50,93,94,95].
Single-botanical studies show clear phenotypic improvements in ageing models. Ginger rhizome extracts, rich in sesquiterpenoids such as zingiberene, reduced biomarkers of senescence and inflammation, increased cellular antioxidant capacity by 15–28%, and extended cellular lifespan [95]. Frankincense (Boswellia serrata (B. serrata)) resin, rich in triterpenoids such as AKBA and KBA, increased free-radical-scavenging activity by up to 80% and suppressed protein glycation and ageing-related damage [93]. Sesquiterpenoid-enriched Kaempferia galanga (K. galanga) ethanolic fractions achieved over 65% inhibition of collagenase and elastase while maintaining fibroblast viability [96]. Diterpenoid-containing coffee extracts, particularly light-roasted Coffea arabica (C. arabica) and medium-roasted C. canephora (C. canephora), exhibited strong antioxidant activity and marked inhibition of collagenase and elastase [97]. Fir-derived terpenes from Abies sibirica (A. sibirica) restored pro-longevity gene-expression patterns in senescent human fibroblasts, suggesting partial reversal of transcriptomic ageing [98].
Complex botanical formulations also provide substantial evidence of terpenoid-associated anti-ageing effects. Ginsenoside-rich ginseng formulations and glycosidic triterpenoids from functional foods increased total antioxidant capacity (T-AOC) by 60–70% and significantly delayed protein and metabolic ageing phenotypes in vivo [94]. Peony-derived preparations containing multiple terpenoid components among over 350 phytochemicals extended C. elegans lifespan by about 18%, improved locomotor performance and stress tolerance, and reduced lipofuscin accumulation [99].
Propolis extracts with high diterpenoid content showed 60–70% inhibition of collagenase and tyrosinase in vitro, highlighting their cell-protective antioxidant effects [100]. Similarly, essential oils from Pulicaria dioscoridis (P. dioscoridis) and Erigeron bonariensis (E. bonariensis), with terpenoid proportions above 93%, concurrently inhibited collagenase, elastase, hyaluronidase, and tyrosinase, and binary combinations outperformed single-source oils across all four enzyme systems [101].
Overall, evidence across cellular, nematode, and mammalian models indicates that terpenoids consistently attenuate biochemical and physiological hallmarks of ageing. Triterpenoids primarily contribute to antioxidant and antiglycation effects, whereas sesquiterpenoids and diterpenoids strongly inhibit extracellular matrix degradation and support cellular resilience, thereby providing robust experimental validation of their anti-ageing relevance (Table 4) [50,100,102].
Table 4. Summary of experimental evidence on terpenoids exhibiting anti-ageing effects across models.
|
Source/Material |
Major Terpenoids |
Experimental Models |
Main Anti-Ageing Outcomes |
References |
|---|---|---|---|---|
|
Ginger (Zingiber officinale)/Frankincense (B. serrata) |
Zingiberene, AKBA, KBA (triterpenoids, sesquiterpenoids) |
Senescent endothelial cells |
↑ SOD, CAT; radical scavenging ↑ to 80%; ↓ protein glycation and oxidative markers; delayed cellular senescence |
|
|
P. dioscoridis, E. bonariensis, K. galanga |
Mono- & sesquiterpenoids (>90%) |
Fibroblast/ECM enzyme inhibition assays |
Collagenase/elastase inhibition ≈ 65–70%; ↓ MMP-1, MMP-3; preserved collagen integrity and matrix viability |
|
|
Coffee extracts (C. arabica, C. canephora)/Greek propolis |
Diterpenoids (e.g., cafestol, kahweol)/diterpenoid-rich chemotypes |
ECM enzyme assays |
Strong collagenase and tyrosinase inhibition (~60–70%); enhanced antioxidant balance and ECM preservation |
|
|
Fir terpenes (A. sibirica)/Ginseng-based formulas |
Terpene complex/Ginsenosides (triterpenes) |
Senescent fibroblasts and rodent models |
↑ AMPK and T-AOC (~60–70%); restored pro-longevity genes and mitochondrial function; ↓ oxidative stress |
|
|
Peony stamen tea/Kuntai capsule |
Mixed terpenoids and multi-type formulations |
C. elegans and rodent ageing assays |
↑ Lifespan (~18%); ↑ SOD and CAT; ↓ oxidative injury; improved ovarian and metabolic function |
Notes: Quantitative changes (↑ increase, ↓ decrease; approximate range 20–40%) summarise representative experimental results across C. elegans, yeast, fibroblast, and rodent models.
3.1.3. Current Evidence of Polyamines for Anti-Ageing
Polyamine supplementation demonstrates consistent anti-ageing efficacy across organisms and tissues. In aged rodents, spermidine (SPD) or spermine (SPM) increased median and maximal lifespan by 10–18% and reduced mortality by up to 35%, while maintaining cardiac, metabolic, hepatic, and neuronal functions [103,104]. Similar benefits have been observed in independent studies, where polyamine administration increased median and maximal lifespan by 10–18% and reduced mortality by up to 35%, with sustained cardiac, metabolic, hepatic, and neuronal functions [105].
Protective effects extend across multiple organ systems. Neuronal and cardiomyocyte ageing models showed approximately 30% improvements in stress resistance, while bone structural integrity was preserved by 10–20% in iron-overloaded aged rats [106]. Age-related inflammation and metabolic decline were likewise alleviated, maintaining systemic resilience in aged animals [34,103].
In vascular and dermal ageing models, angiogenic performance improved by 20–32%, while extracellular-matrix integrity and fibroblast functionality increased by 15–35% [107,108,109]. Consistently, lifespan extension of 8–20% and enhanced stress tolerance were observed in non-mammalian organisms, including C. elegans and honeybees [110,111,112].
Dietary and cellular delivery strategies also produced notable anti-ageing effects. SPD-rich preparations inhibited low-density lipoprotein (LDL) oxidation by approximately 85% and reduced endothelial cytotoxicity by over 60%, supporting vascular protection against ageing [113]. Inhibition of polyamine degradation reduced senescence burden by 20–35% in mammalian cells [114]. Polyamine intervention further mitigated tissue-specific ageing, improving ovarian cell quality by 25–40%, reducing neuronal senescence by 30–50% under hyperglycaemic stress, and attenuating pulmonary ageing-related degeneration by 20–35% [35,115,116].
Overall, current experimental evidence consistently identifies polyamines as multi-organ protective factors that mitigate physiological deterioration and extend organismal health-span across diverse ageing models (Table 5).
Table 5. Summary of experimental evidence on polyamines exhibiting anti-ageing effects across models.
|
Source/Material |
Major Polyamines |
Experimental Models |
Main Anti-Ageing Outcomes |
References |
|---|---|---|---|---|
|
SPD/SPM supplementation in aged rodents |
SPD, SPM |
Aged mice and rats |
↑ Lifespan 10–18%; ↓ mortality 30–35%; improved systemic function |
|
|
SPD in neuronal and bone-ageing models |
SPD |
SAMP8 and iron-overloaded rodents |
↓ Neurodegeneration 30–45%; ↑ behavioural and bone integrity 10–20% |
|
|
SPM in hepatic, cardiac and vascular ageing |
SPM |
Hepatic, cardiomyocyte, and endothelial models |
↓ Inflammation 25–35%; ↑ viability and angiogenesis 20–30% |
|
|
SPD in telomere-, reproductive and systemic ageing |
SPD |
Mouse longevity and porcine oocyte models |
↓ Telomere shortening ≈ 90%; ↑ oocyte integrity ≈ 20%; delayed physical ageing |
|
|
SPD against oxidative and inflammatory ageing |
SPD |
Macrophage, zebrafish, and marrow models |
↓ Oxidative damage 25–40%; ↑ survival and ageing resilience |
|
|
SPD in skin and respiratory ageing |
SPD |
Fibroblast and lung fibrosis models |
↑ ECM integrity 15–28%; ↓ cell loss and degeneration 20–35% |
|
|
Polyamine-rich functional foods (Lycium ruthenicum) |
SPD-containing extracts |
C. elegans longevity assay |
↑ Lifespan 10–20%; ↑ stress tolerance |
[99] |
Notes: Quantitative outcomes (↑ increase; ↓ decrease; % relative change) were extracted from the referenced experimental studies covering biochemical, cellular and rodent ageing models.
3.1.4. Current Evidence of Polysaccharides for Anti-Ageing
Polysaccharides from edible and medicinal sources exhibit consistent anti-ageing efficacy across cellular, nematode, fruit-fly, and mammalian models. Their benefits include lifespan extension, enhanced oxidative defence, improved physiological performance, and attenuation of age-associated tissue degeneration (Table 6).
In cellular ageing systems, Tremella fuciformis polysaccharides increased fibroblast viability and reduced oxidative biomarkers, indicating rejuvenating effects in dermal-ageing models [120]. Fermented ginseng–microbiota extracts decreased intracellular ROS by about 70% and cytosolic superoxide by 30%, demonstrating enhanced bioactivity via microbial transformation [121]. Polysaccharides from Cibotium barometz improved mitochondrial gene expression and muscular integrity, while pomegranate-derived complexes produced a twofold increase in antioxidant indices [72,122].
Across C. elegans models, mushroom polysaccharides such as those from Agaricus bisporus extended lifespan by 10–22% and increased thermotolerance and oxidative-stress resistance through gut–microbiota interactions [123]. L. barbarum polysaccharides improved locomotor activity and stress resilience while reducing intestinal lipofuscin and ROS accumulation [124,125]. Combinations of Cistanche deserticola polysaccharides with probiotics further enhanced antioxidant-enzyme activities and longevity outcomes. Similarly, Chlorella polysaccharides prolonged survival by 14–43%, decreased ROS by 43%, and elevated SOD and CAT activities by 1.2–1.5-fold [126,127]. Other species, including Polygonatum sibiricum (P. sibiricum), Astragalus membranaceus (A. membranaceus), and Codonopsis pilosula (C. pilosula), produced comparable benefits with lifespan extensions of 12–30% [32,128,129].
In D. melanogaster, Tremella polysaccharides delayed age-related declines in climbing ability and oxidative tolerance, while white-tea and Chenopodium quinoa polysaccharides improved endurance, memory, and cognitive performance, indicating neuroprotective effects [130,131].
In mammalian ageing models, particularly D-galactose-induced mice, polysaccharide supplementation consistently increased SOD, CAT, and glutathione peroxidase (GPx) activities by 20–45%, reduced malondialdehyde (MDA) by 25–40%, and improved learning ability and physical performance [32,132,133]. Gut–microbiota restoration was repeatedly observed, characterised by higher probiotic abundance and reduced ageing-associated taxa [134,135]. Dendrobium officinale (D. officinale), Acanthopanax senticosus, O. japonicus, and R. glutinosa polysaccharides preserved tissue integrity, enhanced behavioural and metabolic resilience, while marine and plant-residue polysaccharides supported cardiovascular and dermal protection [136,137,138]. Pleurotus eryngii residues significantly improved skin hydration by 33% and hydroxyproline levels by 46%, confirming structural support [139].
Fermentation and delivery strategies further enhanced efficacy. Fermented Polygonatum and co-formulated polysaccharide–probiotic systems produced 1.5–2.0-fold stronger antioxidant responses and extended lifespan, while nanoparticle delivery improved bioavailability and systemic performance [140,141,142].
Collectively, current evidence identifies polysaccharides as broad-spectrum anti-ageing agents that strengthen oxidative defences, regulate gut–metabolic balance, and preserve multi-organ function across biological systems, thereby extending health-span and delaying functional decline.
Table 6. Summary of experimental evidence on polysaccharides exhibiting anti-ageing effects across models.
|
Source/Material |
Major Polysaccharides |
Experimental Models |
Main Anti-Ageing Outcomes |
References |
|---|---|---|---|---|
|
Medicinal roots (Polygonatum, Rehmannia, Codonopsis, Cistanche, Achyranthes) |
α- and β-glucans, galactomannans, arabinogalactans |
C. elegans, D-gal ageing mice, ageing rats |
Lifespan ↑10–25%; SOD/CAT ↑25–65%; MDA ↓30–50%; cognition & muscle strength improved; gut microbiota restoration |
|
|
TCM herbs & immune-modulatory botanicals (Astragalus, Bupleurum, Notopterygium, Echinopanax) |
Rhamnogalacturonans, heteropolysaccharides |
C. elegans, mice skin fibroblasts, oxidative-stress rodents |
Oxidative stress ↓35–55%; survival under heat tolerance ↑18–40%; ECM integrity maintained |
|
|
Fruits & berries (Lycium, Longan, Watermelon rind, Agrimony, Quinoa) |
Pectic polysaccharides, arabinogalactans, uronic acids |
C. elegans, D-gal mice |
Lifespan ↑12–22%; ROS ↓30–60%; neuronal & cognitive performance improved; gut dysbiosis reversed |
|
|
Mushrooms (Agaricus, Pleurotus, Tremella, Auricularia) |
β-glucans, mannans |
C. elegans, D-gal mice |
Behavioural function improved; SOD ↑20–55%; inflammation ↓; natural ageing delay |
|
|
Edible & medicinal algae (Ulva, Nostoc, Spirulina) |
Sulfated polysaccharides |
D-gal metabolic mice, oxidative cell models |
Glucose metabolism improvement; antioxidant enzymes ↑30–60%; tissue protection ↑ |
|
|
Tea and tea-like plants |
Arabinogalactans, acidic pectins |
D-gal mice, C. elegans |
Age-related decline reduced; gut-brain axis protection; motility & stress survival ↑25–40% |
|
|
Industrial crop residues & fermentation-enhanced polysaccharides |
Modified heteropolymers after microbial transformation |
D-gal mice, C. elegans, in vitro antioxidant |
Antioxidative capacity ↑1.5–2.3-fold; survival ↑15–25%; SCFAs ↑; beneficial microbiota ↑2–3-fold |
|
|
Others with distinctive evidence (Agave, Hemp residue) |
Fructans, cell-wall polysaccharides |
Enzyme & ageing assays |
ROS ↓; tissue ageing delay |
Quantitative anti-ageing outcomes (↑ increase; ↓ decrease; % percentage change; fold change) were extracted from primary experimental studies across C. elegans, mammalian cell models, and ageing rodent systems.
3.1.5. Current Evidence of Fatty Acids for Anti-Ageing
Consistent evidence across multiple biological models indicates that dietary fatty acids delay functional decline and support healthy-ageing outcomes (Table 7). In C. elegans, seed oils enriched in unsaturated fatty acids extended mean lifespan by 12–38% and maintained locomotor performance under ageing-related stress [148]. Structured docosahenaenoic acid (DHA) lipids similarly improved physical resilience in aged nematodes, sustaining mobility during later life stages [149]. Studies in D. melanogaster further demonstrate that DHA-rich microalgal supplementation significantly prolonged lifespan compared with standard diets [150].
Table 7. Summary of experimental evidence on fatty acids exhibiting anti-ageing effects across models.
|
Source/Intervention |
Major Fatty Acids |
Model |
Anti-Ageing Outcome |
References |
|---|---|---|---|---|
|
Trichosanthes seed oil |
UFAs (ALA, LA, OA) |
C. elegans |
Lifespan ↑≈12–38%; locomotion preserved |
[148] |
|
Structured DHA lipids |
DHA |
C. elegans |
Movement capacity maintained; delayed functional decline |
[149] |
|
DHA-rich marine microalgae |
DHA |
Drosophila |
Lifespan significantly prolonged vs. the control diet |
[150] |
|
Fish oil lifelong feeding |
EPA/DHA |
Wistar rats |
Improved survival profile; ↓age-related mortality |
[151] |
|
Long-term DHA dietary supplementation |
DHA |
Telomerase-deficient mice |
Premature ageing prevented; telomere integrity preserved |
[152] |
|
Higher ω-3 dietary intake |
EPA/DHA |
Human adult cohorts |
↓Phenotypic age acceleration |
[153] |
|
Algal ω-3 + EVOO combination |
DHA/EPA + OA |
Aged Wistar rats |
↓Inflammation markers (COX-2/NOX-4); improved lipid balance |
[154] |
|
Hemp seed oil |
PUFAs-rich |
D-gal ageing rats |
Restored gut–metabolic alterations; improved systemic ageing burden |
[155] |
|
Pumpkin seed oil |
PUFAs-rich |
Enzyme-based tissue ageing assays |
↓Collagenase/elastase involved in structural ageing damage |
[156] |
|
Coffee-ground fatty acids |
LA/OA/PA |
Enzyme-based tissue ageing assays |
↓Matrix-degrading enzyme activities |
[157] |
|
Black soybean fatty-acid extracts |
Mixed UFAs |
Food ageing & antioxidant screen |
Antioxidant activity retained in ageing crops |
[158] |
Quantitative anti-ageing outcomes (↑ increase; ↓ decrease; % percentage change) were extracted directly from primary experimental studies across C. elegans, Drosophila, rodent ageing systems, and human nutritional cohorts, encompassing functional longevity measurements, systemic metabolic and inflammatory biomarkers, and enzyme-based tissue-integrity indicators.
In telomerase-deficient mice, prolonged dietary DHA prevented premature ageing phenotypes and helped preserve telomere integrity into adulthood [151]. In telomerase-deficient mice, prolonged dietary DHA prevented premature-ageing phenotypes and preserved telomere integrity into adulthood [152]. The translational relevance of these findings is supported by human cohort data showing that higher dietary eicosapentaenoic acid (EPA)/DHA intake is significantly associated with slower phenotypic-age acceleration across adulthood [153].
Fatty acids also exhibit cardiometabolic support during ageing. A dietary combination of algal ω-3 and extra-virgin olive oil reduced ageing-induced pro-inflammatory protein expression and partially restored circulating lipid profiles toward more youthful compositions in aged rats [154]. These beneficial changes indicate that balanced lipid intake may counteract age-related disturbances in systemic homeostasis.
Plant-derived polyunsaturated fatty acids (PUFAs) sources reinforce these effects across tissues and metabolic domains. Hemp-seed oil significantly improved metabolic signatures and restored dysregulated digestive–lipid interactions in D-galactose-aged rats [155]. Pumpkin-seed oil and fatty-acid extracts from spent coffee grounds suppressed the activity of collagen- and elastin-degrading enzymes associated with structural deterioration [156,157]. Lipid-rich extracts from aged black soybeans retained antioxidative potential despite age-related nutrient loss, highlighting their sustained functional value in later-life nutrition [158].
Collectively, these findings demonstrate a convergent anti-ageing profile of dietary fatty acids across multiple species and biological levels. Improvements in survival, activity maintenance, metabolic regulation, systemic inflammatory balance, and tissue integrity position fatty acids as practical nutritional strategies to attenuate biological ageing and preserve functional health-span across the lifespan.
3.1.6. Current Evidence of Bioactive Peptides for Anti-Ageing
Evidence from nematode, mammalian, and human nutritional models demonstrates that bioactive peptides derived from food proteins produce quantifiable improvements in anti-ageing indicators, primarily reflected in reduced oxidative stress, extended survival, and enhanced functional recovery during ageing (Table 8).
In C. elegans, peptides extracted from Arca subcrenata (A. subcrenata) prolonged lifespan by 18–32%, accompanied by reduced ROS, fat, and lipofuscin accumulation under oxidative challenge [159]. Comparable effects were observed in nematodes treated with peptides derived from Porphyra haitanensis (P. haitanensis), where digested fractions extended lifespan by 15–28% and increased antioxidant-enzyme activity by 1.3–1.5-fold [160]. A newly identified peptide from Arthrobacter ruber (A. ruber) enhanced nematode survival by 25%, improved motility, and reduced oxidative biomarkers during ageing [161]. Soybean-derived peptides also showed pronounced effects across cellular and whole-organism models. In aged nematodes and BALB/c mice, hydrolysed soybean protein increased oxidative resilience by 20%, elevated SOD and CAT activities by 30–40%, and reduced MDA levels by 35–45% [162].
Consistently, antioxidant soybean-peptide fractions exhibited 1.5-fold higher T-AOC and 35–45% lower lipid peroxidation in D-galactose-induced ageing models [163]. At the cellular level, short regulatory peptides such as KED and AEDG reduced β-galactosidase activity by 1.5–2.4-fold and decreased p21 expression by 15%, indicating a measurable delay in cellular-senescence progression [164].
In mammalian systems, fish-collagen peptides enriched with bovine colostrum significantly improved skin firmness and hydration, reducing wrinkle depth by 25–40% and increasing elasticity by over 30% after continued consumption [165]. Extracts derived from sardine waste and codfish frames inhibited matrix-degrading enzymes and down-regulated inflammatory cytokines interleukin-8 (IL-8) decreased 58%; IL-6 decreased 47%), indicating benefits for maintaining dermal structure and delaying visible ageing [166]. Similarly, turtle-derived peptides and their functional derivatives significantly reduced colonic inflammation by 40–60%, restored tight-junction protein levels, and rebalanced gut-microbiota composition toward a youthful, anti-inflammatory profile [167].
Collectively, convergent evidence from nine independent studies confirms that food-derived peptides reproducibly mitigate oxidative and inflammatory damage, extend lifespan, and restore functional and structural integrity across nematode, rodent, and human systems. Quantitative improvements generally range between 15–40% for functional or oxidative indices and 1.3–1.6-fold for antioxidant-enzyme activity, demonstrating their translational potential as safe and efficacious dietary or functional interventions for healthy ageing.
Table 8. Summary of experimental evidence on bioactive peptides exhibiting anti-ageing effects across models.
|
Source/Intervention |
Major Peptide |
Model |
Anti-Ageing Outcome |
References |
|---|---|---|---|---|
|
Marine and seaweed-derived antioxidant peptides (A. subcrenata, P. haitanensis) |
Marine/seaweed peptides |
C. elegans |
Lifespan ↑ ≈ 15–32%; SOD/CAT ↑ 1.3–1.5 fold; ROS and lipofuscin ↓ are significant |
|
|
Bacterial and plant-derived functional peptides (A. ruber, soybean hydrolysates) |
Small molecule & plant peptides |
C. elegans/mice |
Lifespan ↑ 20–30%; SOD/CAT ↑ 30–40%; MDA ↓ 35–45% |
|
|
Antioxidant soybean peptides |
Low-molecular-weight fractions |
Aged mice |
Lipid peroxidation ↓ 35–45%; T-AOC ↑ ≈ 1.5-fold |
[163] |
|
Short regulatory peptides (KED, AEDG) |
Synthetic short peptides |
Senescent cell model |
β-Gal ↓ 1.5–2.4 fold; p21 ↓ ≈ 15%; cell viability restored |
[164] |
|
Marine collagen and residue peptides (fish collagen, sardine/codfish) |
Collagen-derived & marine residue peptides |
Human in vivo/cell models |
Wrinkle depth ↓ 25–40%; elasticity ↑ >30%; IL-8 ↓ 58%; IL-6 ↓ 47%; MMP activity ↓ significant) |
|
|
Turtle peptide (and its derivative) |
Animal-derived functional peptide |
DSS-induced mice |
Colonic inflammation ↓ 40–60%; gut-barrier proteins ↑ are significant |
[167] |
Quantitative anti-ageing outcomes (↑ increase; ↓ decrease; % percentage change; fold change) were extracted directly from primary experimental studies across C. elegans, mammalian cell and rodent ageing models, and human nutritional interventions, encompassing survival, oxidative, inflammatory, and tissue-integrity biomarkers.
3.2. Experimental and Clinical Evidence of Combined Natural Products in Anti-Ageing
Quantitative research from human, animal, and cellular studies consistently demonstrates that interventions combining multiple classes of natural products produce stronger anti-ageing outcomes than single agents. Across seventeen representative studies, formulations incorporating polyphenols, terpenoids, polyamines, polysaccharides, fatty acids, bioactive peptides, amino acids, vitamins, and minerals produced measurable improvements of approximately 15–40% in biomarkers and functional indices associated with ageing (Table 9) [168,169,170].
Large-scale human dietary studies provide robust evidence of this synergistic effect. Long-term adherence to the green Mediterranean diet, which integrates walnuts rich in PUFAs, green tea, and Mankai duckweed as major sources of polyphenols and amino acids, led to pronounced physiological improvements. After 18 months, participants showed a 39% reduction in intrahepatic lipid content and an almost 50% decrease in the prevalence of non-alcoholic fatty liver disease (NAFLD) [170,171]. In parallel, inflammatory and oxidative markers declined by 20–30%, while antioxidant capacity and insulin sensitivity improved. Within the NU-AGE cohort, a Mediterranean-type dietary intervention reversed biological age estimates by approximately 1.5 years and increased metabolic resilience by about 20% [168,172]. Together, these data indicate that dietary combinations rich in omega-3 fatty acids, flavonoids, and amino acids lead to quantifiable reductions in oxidative and metabolic ageing markers, accompanied by measurable epigenetic rejuvenation.
Comparable effects have been reported in animal models treated with composite herbal prescriptions. The Dengzhan Shengmai formula, composed of Erigeron breviscapus (E. breviscapus) flavonoids, Panax ginseng (P. ginseng) saponins, and Ophiopogon japonicus (O. japonicus) polysaccharides, improved learning and memory scores by approximately 30% and reduced inflammatory cytokine levels by 20–40% in D-galactose-induced ageing mice [169]. The Bushen Yizhi and Kaixin San formulas, integrating P. ginseng terpenoids, Polygonum multiflorum (P. multiflorum) polyphenols, and Poria-derived polysaccharides, improved cognitive indices and mitochondrial integrity by 20–30% [173]. Classical tonics such as Zuogui Wan, Yougui Wan, and Gengnian Chun, each containing Rehmannia glutinosa (R. glutinosa), Lycium barbarum (L. barbarum), and Epimedium brevicornum (E. brevicornum), extended Caenorhabditis elegans (C. elegans) lifespan by 20–35% and enhanced motility and antioxidant activity by about 25% [174,175]. Collectively, these studies confirm that combinations enriched in polysaccharides, terpenoid saponins, and polyphenols synergistically maintain neural and metabolic function, typically improving outcomes by 20–35% relative to untreated controls.
Evidence from newer botanical and food-derived complexes further supports this pattern. Enzymatically hydrolysed whole-grain extracts containing polysaccharides, peptides, and phenolic acids extended C. elegans lifespan by approximately 38% and improved tolerance to oxidative and ultraviolet stress by about 35% [176,177]. Similarly, a dual-species green-algae complex rich in polyphenols, peptides, and unsaturated fatty acids enhanced fibroblast viability by 40% and prolonged nematode lifespan by nearly 30% [178]. Multi-herbal preparations such as Liuwei Dihuang and Jianpi Yangwei, which combine R. glutinosa, Cornus officinalis, and Poria cocos with triterpenoids and polysaccharides, yielded mean lifespan gains of 20–30% in nematode and Drosophila melanogaster (D. melanogaster) models [179,180].
Comparable magnitudes of benefit have also been observed in human supplementation and community-based studies. Combined administration of nicotinamide riboside and pterostilbene in older adults reduced oxidative damage markers by approximately 20% and accelerated muscle function recovery by 15% compared with placebo [181]. Observational data from ageing populations in Australia and Japan show that individuals habitually consuming complex mixtures of green tea, soy foods, seaweed, and herbal tonics exhibit approximately 20% higher self-rated health and functional capacity scores [182]. In vitro assays further confirm that mixed extracts of flavonoids and phenolic acids display antioxidant capacities 25–30% greater than those of single components [183].
Taken together, these convergent findings reveal a coherent pattern of interaction across human and experimental systems. The complementary actions of polysaccharides, peptides, and fatty acids reinforce structural integrity and metabolic stability, while polyphenols and terpenoids provide antioxidative and anti-inflammatory modulation. Collectively, these compounds act through interconnected pathways that enhance mitochondrial efficiency, regulate redox balance, and stabilise systemic homeostasis. Such evidence substantiates the mechanistic rationale for multi-class natural-product combinations as an integrative strategy for promoting healthy ageing and preventing age-associated decline.
Table 9. Summary of experimental evidence on combination natural products exhibiting anti-ageing effects across models.
|
Source/Intervention |
Major Components/Classes |
Model |
Anti-Ageing Outcome |
File References |
|---|---|---|---|---|
|
Polyphenol- and fatty-acid-enriched dietary combinations (Green Mediterranean and Mediterranean-type diets) |
Walnuts (PUFAs), green tea and Mankai duckweed (polyphenols and amino acids), olive oil, fish and whole grains (fibre and micronutrients) |
Overweight/elderly human participants |
Intrahepatic fat ↓ ≈ 39%; NAFLD prevalence ↓ ≈ 50%; inflammatory lipids ↓ 20–30%; antioxidant capacity and insulin sensitivity ↑; epigenetic age reversal ≈ 1.5 years after 12–18 months |
|
|
Multi-herbal tonics containing polysaccharides, saponins, and polyphenols (Dengzhan Shengmai, Bushen Yizhi, Kaixin San, Sisheng Bulao) |
E. breviscapus flavonoids (polyphenols), P. ginseng saponins (terpenoids), O. japonicus polysaccharides; plus P. multiflorum polyphenols, Cistanche phenylethanoid glycosides, and other tonic polysaccharides |
D-galactose/SAMP8 ageing mice and related models |
Learning and memory ↑ 25–35%; NGF/BDNF ↑ ≈ 30%; mitochondrial function ↑ ≈ 30%; senescence-associated and inflammatory markers ↓ 20–40% |
|
|
Traditional rejuvenation prescriptions in nematode and fly models (Zuogui Wan, Yougui Wan, Gengnian Chun, Liuwei Dihuang, Jianpi-Yangwei, and related antioxidant formulas) |
Rehmannia, Lycium, Cuscuta, Epimedium, and other roots/fruits rich in polysaccharides, flavonoids (kaempferol, quercetin), terpenoid saponins, and phenolic acids |
C. elegans/Drosophila |
Lifespan ↑ 20–35%; SOD/CAT activity ↑ ≈ 30%; stress resistance ↑ 20–35%; age-related motility declines and lipofuscin accumulation ↓ |
|
|
Cereal- and algae-derived composite extracts (whole-grain hydrolysate and dual green-algae complex) |
Cereal polysaccharides, bioactive peptides, phenolic acids, minerals, together with algal polyphenols, peptides, unsaturated fatty acids, and pigments |
C. elegans/fibroblast systems |
Lifespan ↑ ≈ 30–38%; stress resistance ↑ 30–35%; fibroblast viability ↑ ≈ 40%; collagen degradation ↓ ≈ 25%; ROS and lipofuscin ↓ markedly |
|
|
Combined nutrient supplementation in ageing adults (nicotinamide riboside and pterostilbene) |
Vitamin B3 derivative nicotinamide riboside and stilbene polyphenol pterostilbene |
Elderly humans with experimental muscle injury |
Oxidative-damage biomarkers ↓ ≈ 20%; recovery of muscle strength ↑ ≈ 15% after 3-week supplementation vs. placebo |
[181] |
|
Habitual multi-food dietary patterns in ageing populations (tea, soy, seaweed, herbal tonics) |
Green tea, soy products, seaweed, fruits, vegetables, and herbal tonics provide polyphenols, polysaccharides, fibre, minerals and marine fatty acids |
Older adults in Australia and Japan (observational) |
Self-rated health ↑ ≈ 20%; physical function ↑ ≈ 20%; estimated healthy-life-expectancy indices higher than in low-intake groups |
[182] |
|
Low-grade multi-botanical extracts with synergistic antioxidant activity |
Mixed flavonoids and phenolic acids from pineapple and lime and related botanicals |
In vitro antioxidant and cell-based assays |
T-AOC ↑ 25–30%; lipid peroxidation and protein oxidation ↓; anti-melanogenesis and collagen-biosynthesis stimulation comparable to or approaching positive controls |
[183] |
Quantitative anti-ageing outcomes (↑ increase; ↓ decrease; % percentage change; fold change) are extracted from the primary experimental and clinical studies you provided, across nematode, fly, rodent, and human models, covering oxidative, inflammatory, cognitive, metabolic, and survival-related biomarkers.
3.3. Comparative Anti-Ageing Outcomes Between Single and Combined Natural Products
Quantitative comparisons across human, animal, and cellular models demonstrate that multi-component formulations consistently outperform single-compound interventions in both the magnitude and diversity of anti-ageing outcomes (Figure 3).
Single natural products exhibit moderate but measurable effects. Polysaccharides generally extend lifespan by approximately 15–20% and enhance antioxidant-enzyme activities such as SOD, catalase (CAT), and GPx by 30–35%, accompanied by 25–30% decreases in oxidative and inflammatory markers [44,152,186]. Polyphenols, including catechins, resveratrol, and EGCG, elicit slightly stronger responses, extending C. elegans or rodent lifespan by 18–25%, increasing antioxidant-enzyme activity by 40–45%, and reducing lipid peroxidation by 30–40% [32,62,123]. Bioactive peptides enhance enzymatic defence by approximately 30% and improve cognitive or metabolic indices by 10–20% [187,188,189], whereas fatty acids, terpenoids, and polyamines generally yield smaller yet consistent improvements of 20–30% across redox and inflammatory dimensions [28,163,190].
In contrast, formulations combining several bioactive classes consistently achieve approximately 15–40% higher biochemical or functional gains compared with single components. In murine models, the Dengzhan Shengmai preparation, composed of flavonoids, saponins, and polysaccharides, improved learning and memory by approximately 30% and reduced inflammatory-cytokine levels by 20–40% [173,184]. The Bushen Yizhi composite increased nerve growth factor (NGF) expression by approximately 30% and reduced neuronal apoptosis by about 25% in senescence-accelerated mice [32,33,173,191]. Classical prescriptions such as Zuogui Wan and Yougui Wan, each containing R. glutinosa, L. barbarum, Coptis chinensis, and E. brevicornum, extended C. elegans lifespan by 20–35% and improved motility by approximately 25% [39,174].
Human dietary interventions show parallel effects. Long-term adherence to a green Mediterranean dietary pattern rich in polyphenols, amino acids, and PUFAs reduced intrahepatic lipid content by approximately 39% and decreased the prevalence of NAFLD by about 50% after 18 months, accompanied by 20–30% decreases in circulating inflammatory and oxidative markers [168,172,178], At the cellular level, a cereal–algae composite containing polysaccharides, peptides, and phenolic acids increased C. elegans lifespan by approximately 38%, enhanced oxidative-stress resistance by 35%, and improved fibroblast viability by 40% compared with single components [121,176]. Supplementation with nicotinamide riboside and pterostilbene in older adults reduced oxidative-damage biomarkers by approximately 20% and accelerated muscle-function recovery by about 15% [181]. Observational data from older populations in Australia and Japan further indicate that habitual consumption of mixed green tea, soy foods, seaweed, and herbal tonics corresponds to approximately 20% higher self-rated health and functional capacity than in low-intake groups [182].
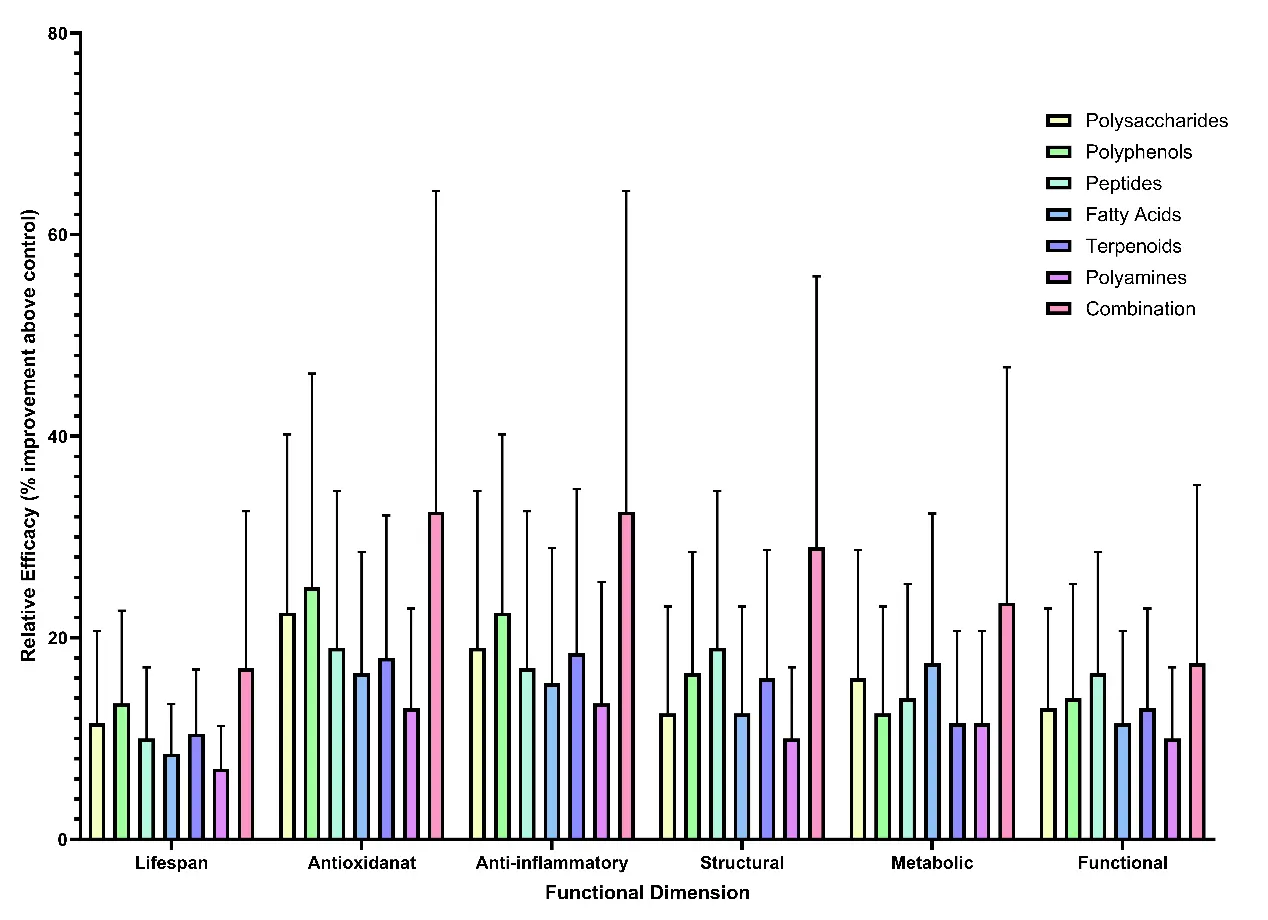
Figure 3. Quantitative comparison of anti-ageing efficacy among different classes of natural compounds and their combinations. Notes: Quantitative comparison of efficacy across six functional dimensions, lifespan, antioxidant, anti-inflammatory, structural, metabolic, and functional, for polysaccharides, polyphenols, peptides, fatty acids, terpenoids, polyamines, and their combinations.
Equation (2). Synergistic Ratio (SR)
|
$$\mathrm{SR} = \frac{\mathrm{E}_{\mathrm{combination}} - \mathrm{E}_{\mathrm{single}}}{\mathrm{E}_{\mathrm{single}}} \times 100\%$$ |
(2) |
Notes: Ecombination is the mean efficacy or biomarker improvement derived from multi-component treatments. Esingle is the mean efficacy or biomarker improvement derived from corresponding single-compound.
Equation (3). Combination Index (CI)
|
```latex\mathrm{C}\mathrm{I}=\mathrm{ }\frac{{\mathrm{d}}_{1}}{{\mathrm{D}\mathrm{x}}_{1}}+\frac{{\mathrm{d}}_{2}}{{\mathrm{D}\mathrm{x}}_{2}}``` |
(3) |
Notes: d1 and d2 are doses in combination; Dx1 and Dx2 are doses required for the same effect individually. CI < 1 indicates synergy, CI = 1 additive, CI > 1 antagonistic.
SR values (Equation (2)), derived from integrated comparisons between combined and single-compound interventions, typically ranged from 25–60%, corresponding to approximately 1.5–2.0-fold greater functional improvement in redox balance, metabolic stability, and cognitive performance. Across datasets, antioxidant-enzyme activities such as SOD, CAT, and GPx increased from 30–35% in single polysaccharide treatments to 50–65% in polysaccharide–polyphenol combinations, while lifespan extension improved from approximately 20–32% under comparable conditions.
The overall interaction intensity among bioactive components, expressed by the CI (Equation (3)), generally ranged from 0.6 to 0.8 across datasets, indicating measurable synergy rather than simple additive accumulation. In this analytical framework, values below one indicate synergy, those near one reflect additivity, and those above one suggest antagonism. The strongest synergistic effects were observed in formulations combining polyphenols with polysaccharides or peptides with PUFAs, highlighting enhanced redox recovery and improved metabolic stability.
Overall, quantitative evidence demonstrates that rationally designed combinations of natural products yield approximately 20–35% greater improvements than individual compounds, enhancing antioxidant defence, anti-inflammatory regulation, metabolic balance, and cognitive performance in a coordinated manner. Taken together, SR and CI analyses quantitatively confirm that multi-class natural-product combinations provide consistently superior anti-ageing efficacy through integrated biochemical reinforcement and systemic adaptation.
4. Mechanistic Basis of Single and Combined Natural Products in Anti-Ageing
4.1. Mechanistic Insights into Single Natural Products
Natural products have long served as a foundational source of biomedical innovation and drug discovery. Their bioactive constituents exert pleiotropic effects that modulate fundamental ageing-related pathways, thereby supporting anti-ageing strategies through antioxidant, anti-inflammatory, and cytoprotective mechanisms [192].
Certain compound classes were excluded owing to insufficient mechanistic evidence or inconsistency with the definition of natural products. These include alkaloids with fragmented or inconclusive data; mineral-derived elements such as Zn and Se; synthetic NAD+ precursors; fungal metabolites such as psilocybin, excluded for ethical and regulatory reasons; and vitamins considered solely as essential nutrients rather than multifunctional bioactives.
Although these classes are discussed individually in the following sections, their molecular targets converge within a vertically organised regulatory hierarchy encompassing epigenetic, redox-inflammatory, and metabolic-energy regulatory layers (Figure 4).
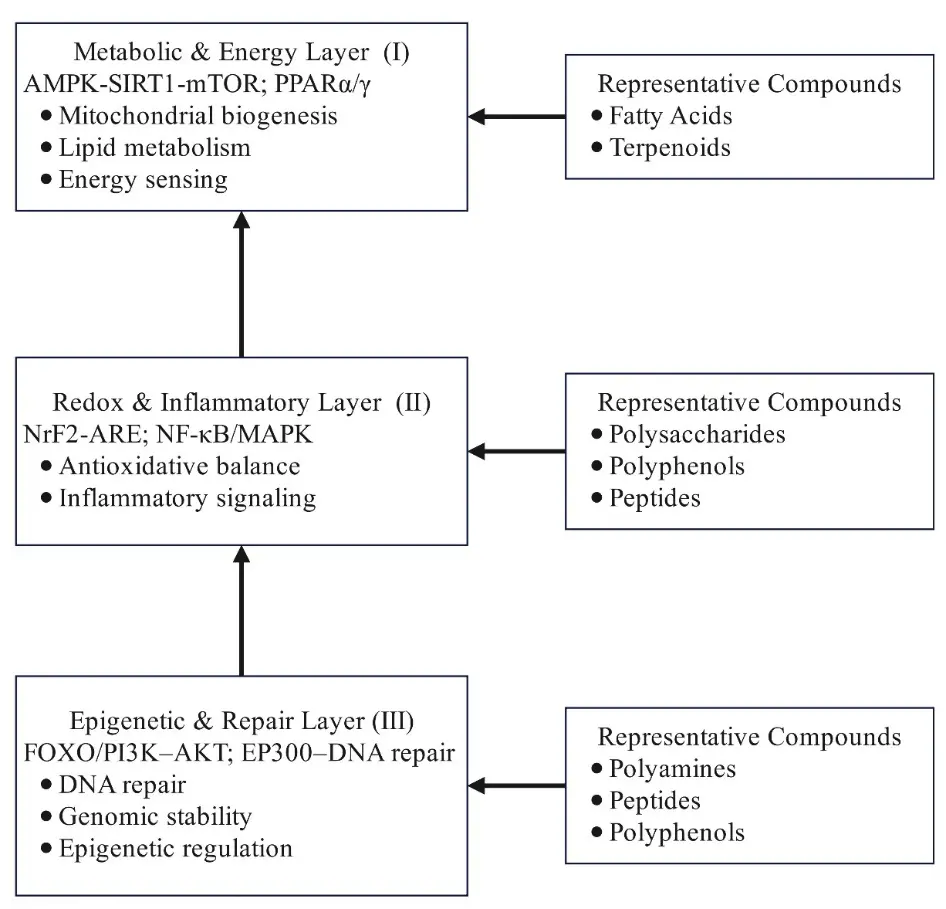
Figure 4. Functional hierarchy of natural products in anti-ageing regulation. Notes: Natural products act through three interconnected layers: epigenetic and repair, redox and inflammatory, and metabolic and energy regulation, forming a coordinated network from genomic maintenance to metabolic activation.
4.1.1. Polyphenols: Mechanistic Basis and Evidence
Polyphenols, a diverse group of plant-derived secondary metabolites encompassing flavonoids, phenolic acids, stilbenes, and lignans, exhibit potent antioxidant and regulatory properties that promote cellular protection and longevity [193,194,195].
Polyphenols directly neutralise ROS through their phenolic hydroxyl groups, which participate in hydrogen atom transfer reactions to generate resonance-stabilised intermediates that terminate oxidative chain reactions [40,42,196]. Beyond this chemical reactivity, they enhance endogenous defences by upregulating SOD, CAT, and GPx activities and by activating the Nrf2–ARE cascade, which induces antioxidant and detoxifying enzymes such as HO-1, NQO1, and glutathione S-transferase (GST) [37,41,197]. In parallel, polyphenols modulate redox-regulatory microRNAs including miR-181b and miR-30c, contributing to stable intracellular redox homeostasis [37,198].
Through SIRT1 activation and suppression of the NF-κB and MAPK cascades, polyphenols attenuate inflammatory gene expression and cytokine release, reducing TNF-α, interleukin-1β (IL-1β), and IL-6 production while maintaining endothelial nitric oxide synthase (eNOS) activity and nitric oxide bioavailability [36,38,199]. Inhibition of COX-2 expression and arachidonic acid (AA) metabolism further constrains chronic inflammatory signalling, collectively mitigating inflammageing and supporting immune equilibrium [36,38,199].
Polyphenols preserve mitochondrial integrity by activating the AMPK–SIRT1–PGC-1α axis, which enhances mitochondrial biogenesis and respiratory efficiency while promoting autophagic clearance of damaged organelles. Upregulation of nuclear respiratory factor 1 (NRF1), TFAM, and microtubule-associated protein 1 light chain 3 (LC3)-II maintains mitochondrial dynamics and ATP production, reducing oxidative stress and delaying senescence [193,200,201]. By suppressing mTORC1 and inducing enhancing transcription factor EB (TFEB) nuclear translocation, polyphenols facilitate lysosomal biogenesis and autophagic flux, linking nutrient sensing to cellular renewal and proteostasis [60,61,202].
At the systemic level, polyphenols influence the gut–microbiota–immune network by enriching beneficial taxa such as Bifidobacterium, Lactobacillus, and Akkermansia while reducing pro-inflammatory genera including Enterobacteriaceae and Proteobacteria [59,203]. These compositional changes enhance short-chain fatty acid synthesis and improve mucosal barrier integrity by upregulating Muc2 and tight-junction proteins such as occludin and ZO-1, thereby reinforcing intestinal homeostasis and lowering systemic inflammation [60,204,205].
4.1.2. Terpenoids: Mechanistic Basis and Evidence
Terpenoids, a structurally diverse class of natural compounds derived from isoprene units, are one of the most abundant families of bioactive metabolites in plants, fungi, and marine organisms. They display wide-ranging pharmacological activities, including antioxidant, anti-inflammatory, and neuroprotective actions, which collectively underpin their importance in anti-ageing research [206,207,208].
At the mechanistic level, terpenoids exert anti-ageing effects by regulating oxidative balance, inflammation, mitochondrial homeostasis, and cellular repair. Carotenoids, a subclass of tetraterpenoids, quench singlet oxygen by absorbing its excess energy and dissipating it as heat, thereby interrupting oxidative chain reactions without structural degradation. They selectively react with radicals such as NO2·, RS·, and RSO2· through electron transfer and radical addition, forming resonance-stabilised intermediates that decay into non-radical products and maintain sustained antioxidant protection [206,209].
Triterpenoids such as ursolic acid, lupeol, and ginsenosides act as potent regulators of longevity-related pathways, including mTORC1/AKT/PI3K, AMPK, SIRT1, MAPK, FOXO, NRF2, and NF-κB. These compounds enhance endogenous antioxidant defences by restoring SOD, CAT, GPx, and GST activities, elevating glutathione levels, and reducing tissue oxidative injury [48,210]. Activation of the SIRT1/sirtuin 6 (SIRT6) axis in the hypothalamus improves metabolic regulation and energy balance, while modulation of PGC-1α/peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC-1β) signalling promotes mitochondrial biogenesis and oxidative phosphorylation. These processes collectively sustain mitochondrial integrity and energy metabolism, with concomitant increases in α-Klotho protein (Klotho) expression providing systemic protection against age-related metabolic dysregulation [190,191,211].
In addition to mitochondrial regulation, terpenoids activate AMPK while inhibiting mTOR signalling, thereby inducing autophagy and metabolic reprogramming. This dual modulation enhances the clearance of damaged proteins and mitochondria, restores homeostasis, and promotes cellular repair [191]. By suppressing PI3K/AKT and ERK/p38 phosphorylation, terpenoids improve neural stem-cell function and attenuate senescence-associated impairments [211]. Lupeol further delays cellular senescence by downregulating p53, p21, and p16 expression, reducing senescence-associated β-galactosidase activity, and suppressing matrix metalloproteinases (MMPs) such as MMP-1, MMP-2, and MMP-3 overexpression, thereby maintaining extracellular-matrix integrity and delaying photoaging [212].
Diterpenoids and triterpenoids also engage PI3K/AKT–NF-κB and PI3K/p38–Nrf2–HO-1 pathways to provide neuroprotection and cryoprotection. Activation of Nrf2 and HO-1 reduces oxidative injury and enhances neuronal survival under metabolic or oxidative stress [212,213]. Concurrent suppression of NF-κB and modulation of MAPK/ERK/p38 signalling alleviate chronic inflammation, stabilise redox balance, and preserve tissue homeostasis [214].
Collectively, these findings identify terpenoids as multifunctional modulators of ageing-related signalling networks. Through integrated control of AMPK–SIRT1–mTORC1, Nrf2–ARE, and PI3K–AKT–NF-κB cascades, terpenoids coordinate antioxidant defence, autophagy, and energy metabolism, thereby promoting neuroprotection, tissue repair, and metabolic resilience. Their multitarget actions and cross-pathway regulation position terpenoids as key natural agents for mitigating ageing and age-associated degenerative disorders.
4.1.3. Polyamines: Mechanistic Basis and Evidence
Polyamines are small polycationic alkylamines containing two or more amino groups (−NH3+) and are primarily synthesised from L-ornithine via amino-acid decarboxylation. Their positive charges enable interactions with negatively charged biomolecules such as DNA, RNA, ATP, and phospholipids, thereby regulating nucleic acid conformation, ion balance, and protein synthesis [215,216]. Putrescine, SPD, and SPM are the principal forms, whereas non-canonical species such as cadaverine and norspermidine occur predominantly in prokaryotes. Post-synthetic acetylation and methylation influence intracellular localisation and metabolic stability [217,218]. Functionally, polyamines act as endogenous bioactive molecules with antioxidant, anti-inflammatory, and regulatory activities that preserve redox balance, immune stability, and gut barrier function. Elevated polyamine levels, particularly SPD, correlate with extended lifespan and improved physiological resilience across model organisms, and altered polyamine metabolism is consistently linked to neurodegenerative and cardiovascular ageing [219,220].
At the molecular level, polyamines exert antioxidant effects through metal chelation and membrane stabilisation. Their polycationic nature binds transition metals such as Fe2+ and Cu2+, preventing hydroxyl radical generation and limiting propagation of lipid peroxidation [221,222]. SPD and SPM directly neutralise hydroxyl radicals and singlet oxygen, enhancing mitochondrial integrity and energy metabolism while lowering ROS accumulation [221,223]. SPM also protects lipid-soluble antioxidants such as α-tocopherol and carotenoids, prolonging vitamin E stability and preserving pigment integrity [224]. These actions collectively contribute to the maintenance of mitochondrial homeostasis and oxidative-stress defence, supporting cellular longevity and resilience.
Polyamines regulate autophagy and epigenetic signalling that underpin cellular renewal. SPD reduces excessive acetylation and stimulates autophagic flux through upregulation of LC3, Beclin-1, and p62, while stabilising acetylated eIF5A to enable efficient translation of TFEB, a transcription factor governing lysosomal biogenesis and autophagy genes [225,226]. Depletion of polyamines impairs proteostasis and energy balance, whereas supplementation restores autophagic capacity across yeast, nematode, and mammalian models [221]. SPD also inhibits the histone acetyltransferase E1A-associated protein p300 (EP300), inducing chromatin hypoacetylation that stabilises gene expression and supports lifespan extension [225,226]. Regulation of transcription factors such as c-Myc, c-Fos, and c-Jun further maintains cell-cycle control and genomic integrity [227,228].
At the immune and inflammatory interface, polyamines suppress NF-κB p65 translocation and attenuate PI3K–AKT and MAPK activation, reducing inducible nitric oxide synthase (iNOS) and COX-2 expression and the subsequent production of NO, prostaglandin E2 (PGE2), and cytokines such as TNF-α, IL-1β, and IL-6 [229]. They increase interleukin-10 (IL-10) release and decrease leukocyte adhesion by downregulating LFA-1/CD11a on mononuclear cells [220,230,231]. These immuno-modulatory actions cooperate with their antioxidant activity to limit chronic inflammation and preserve cellular homeostasis.
Within the gut–microbiota axis, intestinal bacteria convert ornithine into putrescine and subsequently into SPD and SPM via aminopropyl transfer reactions [219,232]. These metabolites are absorbed by the intestinal epithelium and distributed systemically [233]. In the intestinal mucosa, polyamines stimulate epithelial renewal, enhance tight-junction protein expression, and strengthen barrier integrity [220,234]. They also promote the growth of beneficial microbes and optimise microbial communication through biofilm and vesicle signalling, thereby contributing to metabolic stability and health-span maintenance [232,235].
In summary, polyamines integrate antioxidant, autophagic, epigenetic, and anti-inflammatory mechanisms to sustain cellular and systemic homeostasis. Polyamines coordinate mitochondrial protection, chromatin remodelling, and gut–immune balance, functioning as versatile molecular mediators that collectively delay ageing and enhance physiological resilience.
4.1.4. Polysaccharides: Mechanistic Basis and Evidence
Polysaccharides are complex carbohydrate polymers composed of long chains of monosaccharides joined by glycosidic bonds, exhibiting remarkable structural and functional diversity that underpins their biological activities [236,237]. They may occur as homopolysaccharides containing identical sugar residues, or as heteropolysaccharides composed of mixed monosaccharides such as glucose, galactose, mannose, arabinose, and xylose, arranged in linear or branched configurations [238]. Depending on charge characteristics, they occur as anionic or cationic polymers [239]. The versatile molecular conformations and reactive hydroxyl, sulphate, and amino groups of polysaccharides confer broad biological functionality, allowing them to participate in immune regulation, cellular adhesion, wound repair, and metabolic homeostasis [240,241].
Bioactive polysaccharides from terrestrial and marine sources demonstrate broad anti-ageing potential through coordinated redox regulation, metabolic adaptation, and immune modulation. They enhance antioxidant defence by activating the Nrf2–ARE pathway, increasing SOD, CAT, GPx, and GST expression, and reducing free radical propagation [30,44,242]. Many preparations scavenge hydroxyl and superoxide radicals and inhibit NO overproduction by downregulating iNOS mRNA, thereby decreasing lipid peroxidation and preserving mitochondrial integrity [44,243].
At the metabolic level, polysaccharides adjust energy homeostasis through AMPK–SIRT1–PGC-1α signalling and suppress mTORC1/ribosomal protein S6 kinase (S6K) activity, thereby improving mitochondrial biogenesis, autophagy, and stress tolerance [28,30,39]. In parallel, they modulate the IIS–FOXO–p53 cascade to induce stress-response genes and upregulate DNA-repair enzymes such as 8-oxoguanine DNA glycosylase (OGG1), X-ray repair cross-complementing protein 1 (XRCC1), and poly (ADP-ribose) polymerase 1 (PARP1), thereby reducing phosphorylated H2A histone family member X (γH2AX) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) accumulation and maintaining genomic stability [28,39]. Polysaccharides further promote autophagy and mitophagy via AMPK–SIRT1–Unc-51-like autophagy-activating kinase 1 (ULK1) and PTEN-induced putative kinase 1 (PINK1)/E3 ubiquitin-protein ligase Parkin (Parkin) pathways, supporting telomere maintenance by enhancing telomerase activity and limiting telomere-associated damage [30,244].
Beyond intracellular protection, polysaccharides exert pronounced immunomodulatory and microbiota-regulating effects. Their chain conformation and monosaccharide composition determine interaction with innate immune receptors such as toll-like receptor 2 (TLR2) and toll-like receptor 4 (TLR4) and dendritic cell-associated C-type lectin-1 (Dectin-1), leading to balanced activation of NF-κB and MAPK signalling that reduces IL-6, TNF-α, and IL-1β release while elevating IL-10 production [49].
In the gut, polysaccharides are fermented to short-chain fatty acids (SCFAs) (acetate, propionate, and butyrate) that bind G protein-coupled receptor 41 (GPR41), G protein-coupled receptor 43 (GPR43), and Hydroxycarboxylic acid receptor 2 (HCAR2), enhancing interleukin-18 (IL-18) secretion and reinforcing mucosal immunity [58,245]. They simultaneously strengthen the intestinal barrier by upregulating antimicrobial peptides, mucins, and tight-junction proteins while lowering lipopolysaccharide (LPS) production and epithelial permeability [246,247]. As fermentable substrates, polysaccharides selectively stimulate beneficial taxa such as Akkermansia and Lactobacillus, while suppressing pathogens and reshaping microbial metabolism toward increased SCFAs and reduced pro-inflammatory metabolites (trimethylamine N-oxide (TMAO), indoles), thereby supporting intestinal and systemic equilibrium [49,248].
Sulphated or uronic-acid-rich polysaccharides display strong anti-glycation activity by inhibiting advanced glycation-end-product (AGE) formation and associated oxidative stress [47,249]. Their sulphate and hydroxyl groups trap reactive carbonyl species (RCS) such as methylglyoxal and glyoxal, preventing protein cross-linking, while their intrinsic antioxidant capacity further suppresses AGE–receptor for advanced glycation end-products (RAGE)-mediated inflammation [250,251]. By lowering lipid peroxidation, improving SOD, CAT, and GSH-Px activities, and stabilising mitochondrial membranes, polysaccharides preserve extracellular matrix structure and cellular viability.
Collectively, these actions define polysaccharides as multifunctional macromolecules that integrate redox regulation, metabolic balance, immune synchronisation, and gut-microbiota symbiosis to mitigate oxidative, inflammatory, and glycation-related ageing processes, thereby maintaining systemic homeostasis and promoting longevity.
4.1.5. Fatty Acids: Mechanistic Basis and Evidence
Fatty acids constitute the fundamental structural units of complex lipids that support cellular energy storage, membrane organisation, and signalling regulation. Their structural diversity, defined by chain length and degree of unsaturation, determines their physicochemical properties and biological functions [53,252].
Among them, unsaturated fatty acids, particularly long-chain monounsaturated and polyunsaturated species, are essential for maintaining metabolic flexibility, mitochondrial efficiency, and redox balance. Humans rely on dietary intake of essential fatty acids such as linoleic (ω-6) and α-linolenic acids (ω-3), which serve as precursors for long-chain derivatives including AA, EPA, and DHA [54].
Functionally, fatty acids act as metabolic fuels, membrane constituents, and precursors of bioactive mediators such as eicosanoids. Unsaturated fatty acids, especially monounsaturated fatty acids (MUFAs) and PUFAs, modulate transcriptional programs through PPAR, SIRT1, and AMPK signalling, thereby promoting β-oxidation, mitochondrial biogenesis, and antioxidant defence. Long-chain ω-3 PUFAs such as EPA and DHA exert neuroprotective and cardioprotective effects, preserve telomere length, and correlate with improved longevity indices, whereas MUFAs enhance metabolic stability and membrane fluidity [52,53,57]. Collectively, these features position unsaturated fatty acids as central regulators of energy metabolism, oxidative homeostasis, and cellular lifespan.
Omega-3 fatty acids function as transcriptionally active ligands of PPARs, particularly PPAR-α and PPAR-γ, which regulate lipid oxidation, glucose utilisation, and inflammatory tone. Upon receptor binding, EPA and DHA form heterodimers with the retinoid X receptor (RXR) to activate peroxisome proliferator response elements (PPREs), enhancing fatty-acid oxidation and suppressing NF-κB- and activator protein-1 (AP-1)-mediated cytokine transcription. This signalling modulation increases the expression of antioxidant enzymes such as SOD and CAT, reduces lipid peroxidation, and stabilises cellular redox status. Concurrently, EPA and DHA serve as precursors to specialised pro-resolving mediators (SPMs), including resolvins, protectins, and maresins, which bind formyl peptide receptor 2 (FPR2)/lipoxin A4 receptor (ALX) and G protein-coupled receptor 120 (GPR120) to promote M2 macrophage (M2) polarisation, accelerate efferocytosis, and resolve inflammation [253,254]. Through this dual PPAR–SPM axis, omega-3s convert pro-inflammatory signalling into pro-resolving cascades, mitigating oxidative injury and chronic inflammation that drive cellular ageing [255,256].
Epigenetic and multi-omics studies reveal that ω-3 PUFAs influence DNA methylation and histone acetylation patterns, particularly in genes governing metabolic and immune regulation, such as TNF-α, Interferon alpha-13 (IFNA13), and ATPase phospholipid transporting 8B3 (ATP8B3), leading to reduced inflammatory transcription and improved insulin sensitivity [55]. These changes are tissue-specific yet consistently associated with enhanced metabolic resilience and lower frailty indices. Upregulation of antioxidant systems, including SOD, CAT, GPx, and glutathione, further reduces oxidative modification of proteins and lipids, preventing telomere attrition and genomic instability [254,257]. Diets with higher PUFAs and MUFAs content and a low n-6:n-3 ratio correlate with longer telomeres and slower physiological decline, whereas saturated-fat-rich diets are associated with elevated oxidative stress and frailty [253,258].
Beyond cellular and genomic regulation, ω-3 PUFAs maintain systemic homeostasis through the gut–mucosal–immune axis. Supplementation enhances microbial α-diversity, enriches Bacteroidetes, Akkermansia, and butyrate-producing Lachnospiraceae, and suppresses pro-inflammatory Proteobacteria and Enterobacteriaceae [259,260]. These compositional shifts elevate SCFAs such as butyrate and propionate, which activate G-protein-coupled receptors free fatty acid receptor 2 (FFAR2) and free fatty acid receptor 3 (FFAR3) and inhibit histone deacetylases, reducing pro-inflammatory cytokine expression. SCFAs also serve as energy substrates for colonocytes and promote tight-junction integrity via upregulation of occludin and claudin [258]. Additionally, omega-3s increase intestinal alkaline phosphatase activity, detoxifying LPS and lowering endotoxemia. Omega-3-derived SPMs further enhance mucosal repair and barrier stability, limiting microbial translocation and chronic low-grade inflammation [257,258].
Collectively, these effects reinforce epithelial and immune equilibrium, suppress NF-κB and NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) activation, and preserve mitochondrial function. Through these integrated mechanisms, omega-3 fatty acids sustain metabolic and inflammatory balance, supporting health-span and mitigating age-related decline.
4.1.6. Bioactive Peptides: Mechanistic Basis and Evidence
Bioactive peptides are short amino-acid fragments produced through enzymatic hydrolysis, microbial fermentation, or physiological digestion of proteins, functioning as regulatory molecules that support systemic homeostasis and delay ageing [187,261]. Their activity largely depends on sequence composition and the presence of hydrophobic, proline, lysine, or arginine residues, which influence structural stability and receptor affinity [262]. These peptides originate from diverse sources, including milk, eggs, marine organisms, plants, and microbial-fermentation systems, and exhibit multifunctional bioactivities such as antioxidant, anti-inflammatory, and immunomodulatory effects [261,263]. Peptides derived from marine and fermented sources are particularly notable for their strong metabolic and redox regulatory capacity, supporting cellular resilience and longevity-related pathways.
The anti-ageing actions of bioactive peptides arise from interconnected antioxidant, anti-inflammatory, antiglycation, and metabolic-regulatory mechanisms [187,264]. By scavenging reactive oxygen and nitrogen species and chelating transition metals such as Fe2+ and Cu2+, peptides suppress Fenton-type oxidative reactions and limit lipid peroxidation [189,265]. This redox modulation decreases inhibitor of NF-κB α (IκBα) phosphorylation, blocks NF-κB nuclear translocation, and down-regulates MAPK cascades involving p38, JNK, and ERK [266]. Consequently, the expression of COX-2, iNOS, TNF-α, IL-1β, and IL-6 is attenuated, accompanied by reduced MMP and elastase activities that preserve extracellular-matrix integrity and tissue elasticity [265,267]. This integrated regulation of oxidative and inflammatory pathways enhances cytoprotective capacity, maintains redox equilibrium, and mitigates stress-induced cellular ageing.
At the transcriptional level, bioactive peptides activate the Nrf2–ARE signalling pathway by modifying cysteine residues on kelch-like ECH-associated protein 1 (Keap1), facilitating Nrf2 nuclear translocation and induction of antioxidant enzymes including HO-1, NQO1, and glutamate–cysteine ligase catalytic subunit (GCLC) [29,187]. The enhanced enzymatic capacity restores redox balance and indirectly suppresses pro-inflammatory transcription factors. Parallel modulation of the AMPK–SIRT1–PGC-1α axis promotes mitochondrial biogenesis, enhances β-oxidation, and maintains autophagic flux while repressing mTOR activity [264,268]. The interplay between Nrf2-mediated antioxidation and AMPK–SIRT1 metabolic control forms a coordinated defence circuit that supports mitochondrial integrity, energy efficiency, and proteostasis balance during ageing.
Peptides also prevent carbonyl stress and maintain protein functionality by reducing AGE accumulation. Carnosine and related dipeptides neutralise RCS such as methylglyoxal and MDA, disrupting the AGE–RAGE feedback loop that amplifies oxidative and inflammatory damage [269,270]. This antiglycation effect preserves enzymatic activity and prevents cross-linking of structural proteins, thereby contributing to tissue elasticity and metabolic stability [261]. Through regulation of the AMPK–mTORC1 pathway, peptides further stimulate autophagy and lysosomal degradation of damaged proteins, maintaining cellular-clearance mechanisms and promoting long-term viability [264,271]. These combined antioxidative, antiglycative, and proteostasis functions ensure the preservation of macromolecular integrity under chronic stress.
Beyond intracellular protection, bioactive peptides exert profound effects on immune and vascular systems. By inhibiting NF-κB and JAK/STAT signalling, they promote macrophage transition from the pro-inflammatory M1 macrophage (M1) phenotype to the reparative M2 macrophage (M2) state, reducing cytokine release and restoring immune equilibrium [189,272]. At the endothelial level, activation of the PI3K/AKT/eNOS and vascular endothelial growth factor (VEGF)/fibroblast growth factor (FGF) pathways enhances nitric-oxide synthesis and angiogenic signalling, improving microvascular circulation and oxygen delivery [261,266]. These vascular effects reinforce nutrient exchange and metabolic efficiency, counteracting age-related hypoxia and tissue decline.
Collectively, through integrated control of oxidative defence, autophagy, protein-quality maintenance, immune regulation, and vascular renewal, bioactive peptides establish a multifaceted molecular framework that preserves cellular homeostasis and extends health-span.
4.2. Integrated Mechanistic Framework of Combined Natural Products in Anti-Ageing
The synergistic anti-ageing efficacy of combined natural products arises from the integration of multiple regulatory cascades that collectively sustain oxidative defence, metabolic balance, and cellular repair. Rather than acting through a single dominant route, polyherbal and multi-nutrient formulations engage complementary molecular targets within interconnected pathways such as AMPK–SIRT1–mTORC1, Nrf2–ARE, NF-κB, and PI3K–AKT–MAPK, thereby reinforcing mitochondrial homeostasis, genomic stability, and stress adaptation [188,189,261,268].
4.2.1. Convergent Core Pathways and Shared Mechanistic Networks
The anti-ageing potential of diverse natural product classes arises from their interactions with conserved molecular networks that jointly regulate energy metabolism, redox balance, inflammation, and genomic maintenance. Despite their structural heterogeneity, polysaccharides, polyphenols, peptides, fatty acids, terpenoids, and polyamines converge on overlapping regulatory axes, including AMPK–SIRT1–mTORC1, Nrf2–ARE, NF-κB, FOXO, PPAR, and insulin-like growth factor (IGF)–IIS/PI3K–AKT–MAPK pathways, which together sustain cellular homeostasis and resilience under stress [28,44,188]. This convergence underscores a shared molecular infrastructure through which natural compounds influence longevity-related signalling and adaptive cellular responses [30,189].
At the metabolic level, the AMPK–SIRT1–mTORC1 cascade functions as a central rheostat linking nutrient status with mitochondrial bioenergetics and autophagic renewal. Activation of AMPK promotes SIRT1-mediated deacetylation of PGC-1α and mitochondrial transcription factor A (TFAM), enhancing mitochondrial biogenesis, β-oxidation, and autophagic flux while suppressing mTOR signalling [39,258]. Polyphenols and terpenoids modulate this network via AMPK phosphorylation and SIRT1 activation, whereas fatty acids and polyamines regulate energy metabolism through PPARα/γ–AMPK coupling [190,191].
Redox homeostasis represents a unifying target, primarily mediated through the Nrf2–ARE pathway. Polyphenols, terpenoids, and polysaccharides enhance Nrf2 translocation and transcription of antioxidant enzymes such as SOD, CAT, and HO-1, thereby reducing oxidative stress and lipid peroxidation [187,189]. Fatty acids and peptides contribute indirectly through PPARs- and SIRT1-dependent redox regulation, while polyamines stabilise oxidative–inflammatory equilibrium via reciprocal regulation of Nrf2 and NF-κB [44,273].
At the genomic level, FOXO transcription factors act as conserved effectors of stress resistance and longevity. SIRT1-dependent deacetylation of FOXO1/3a activates repair-related genes such as OGG1 and PARP1, maintaining genomic integrity [28]. Terpenoids and polyphenols modulate FOXO activity through MAPK/ERK-mediated phosphorylation and PI3K–AKT inhibition, thereby supporting autophagic capacity [210]. Concurrently, PPAR and MAPK signalling integrate lipid metabolism with immune regulation, while polyamines couple metabolic sensing with epigenetic stability [233].
Collectively, these mechanisms delineate a shared mechanistic foundation across natural-product categories. Through coordinated regulation of energy sensing, oxidative defence, and genomic stability, these compounds sustain cellular adaptability and stress tolerance. This convergence defines the molecular basis for their anti-ageing efficacy and establishes the platform upon which complementary mechanisms can further integrate [44,188].
4.2.2. Complementary and Parallel Mechanisms of Natural Product Classes
Across the six principal categories of natural products, anti-ageing efficacy is mediated through distinct yet interrelated molecular hierarchies. These include immunometabolism modulation, transcriptional reprogramming, and chromatin-level regulation that operate in complementary and parallel manners to sustain systemic homeostasis [28,33,44]. Each compound class contributes through its characteristic biochemical interface, engaging unique regulatory tiers while maintaining dynamic interactions within shared signalling networks [188,189].
Polysaccharides primarily operate along the immune–microbial axis, modulating the gut-associated lymphoid tissue and commensal microbiota to restore mucosal tolerance and barrier integrity. Astragalus and Ganoderma lucidum polysaccharides attenuate TLR2/4–myeloid differentiation primary response 88 (MyD88)–NF-κB activation in intestinal epithelial and myeloid cells, suppressing transcription of pro-inflammatory genes such as iNOS, COX-2, and TNF-α, while enhancing IL-10 and transforming growth factor-β (TGF-β) expression via signal transducer and activator of transcription 3 (STAT3)-regulated anti-inflammatory signalling [185,274]. Simultaneously, they promote secretory immunoglobulin A (IgA) and short-chain fatty acid production, reinforce epithelial tight-junction integrity, and mitigate microbial translocation. Sulphated marine polysaccharides such as fucoidan further stabilise intestinal epithelia, reduce LPS burden, and inhibit inflammasome activation, thereby attenuating systemic inflammation and metabolic stress [28,44]. Through these mechanisms, polysaccharides function as immunometabolism gatekeepers linking mucosal stability to organismal longevity.
Polyphenols exert broad transcriptional and epigenetic control over inflammatory and oxidative responses. Compounds such as curcumin, resveratrol, and catechins covalently modify Keap1 cysteine residues, releasing Nrf2 to upregulate HO-1, NQO1, and GCLC, while simultaneously suppressing IκB kinase β (IKKβ) and NF-κB p65 nuclear translocation. These effects collectively reduce the expression of IL-6, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) in macrophages and senescent fibroblasts [7,275]. Polyphenols further reprogram histone acetylation through SIRT1 activation and regulate non-coding RNAs such as miR-146a and miR-21, which target TNF receptor–associated factor 6 (TRAF6) and IKKβ, thereby attenuating inflammatory feedback at both transcriptional and post-transcriptional levels [61]. Activation of the SIRT1–AMPK–PGC-1α cascade enhances mitochondrial biogenesis and ATP production, while Nrf2–p62 crosstalk strengthens antioxidant defence and autophagic recycling [188,275].
Bioactive peptides function as signalling modulators that intercept phosphorylation cascades and receptor-mediated cytokine release. Peptides derived from Spirulina platensis, soy, and milk hydrolysates suppress MAPK members, including p38, ERK1/2, and JNK, thereby reducing COX-2 expression and AP-1 activation in immune and stromal cells [187,261,262]. Certain food-derived peptides bind to TLR2 or ACE2 receptor sites, in animal models, peptide supplementation elevates SIRT1 and FOXO3a expression, enhances antioxidant-enzyme activities, and reduces cytokine release, demonstrating dual regulation of redox and inflammatory homeostasis. Additionally, antiglycation peptides neutralise RCS and inhibit MMPs, thereby preserving extracellular-matrix architecture and vascular elasticity [29,187,263].
Fatty acids primarily act through PPAR-centred transcriptional regulation and lipid-mediator resolution. Long-chain ω-3 PUFAs, including EPA and DHA, are enzymatically converted into SPMs that limit neutrophil infiltration, enhance macrophage efferocytosis, and promote M1 to M2 phenotypic transition. Activation of PPAR-α and PPAR-γ further suppresses NF-κB- and AP-1-driven cytokine transcription while promoting the resynthesis of IκBα, thereby maintaining a controlled inflammatory tone [255,256,260]. These fatty acids integrate into membrane phospholipids, alter receptor clustering and lipid-raft composition, and thereby modulate TLR4 signalling while improving redox–metabolic stability [52,259].
Terpenoids exhibit dual redox and metabolic regulation. Carotenoids, ginsenosides, and triterpenes activate Nrf2-dependent antioxidant enzymes while inhibiting IKKβ-mediated NF-κB translocation, thereby coupling oxidative defence with anti-inflammatory control [48,191,210]. They simultaneously modulate AMPK–mTORC1–ULK1 signalling to enhance autophagic flux and upregulate hypothalamic SIRT1–SIRT6–Klotho pathways that maintain nutrient sensing and neuroendocrine balance [190,212,214].
Polyamines, represented by SPD and SPM, act predominantly at the chromatin level. Their positive charge facilitates interaction with DNA and histones, neutralising promoter regions of inflammatory genes and regulating histone acetyltransferase and deacetylase activity [274,276]. SPD promotes autophagic flux via EP300 inhibition and stabilisation of acetylated Eukaryotic translation initiation factor 5A (eIF5A), while TFEB translation and lysosomal biogenesis. These actions suppress NLRP3 inflammasome activity and IL-1β maturation, thereby maintaining proteostasis and genomic stability [216,277].
Collectively, these complementary mechanisms form a stratified anti-ageing hierarchy: polysaccharides preserve immune and microbial balance; polyphenols and terpenoids modulate transcriptional and redox regulation; peptides and fatty acids coordinate cytoplasmic kinase and lipid-mediated signalling; and polyamines maintain chromatin and epigenetic stability. The interaction among these regulatory strata sustains metabolic coordination, oxidative equilibrium, and transcriptional fidelity, providing the molecular foundation for synergistic anti-ageing outcomes [28,44,188].
4.2.3. Hierarchical Integration and Network-Level Synergistic Regulation
The anti-ageing potential of combined natural products arises not merely from additive actions but from the hierarchical coupling of regulatory networks that coordinate metabolic, redox, inflammatory, and genomic processes. This integration represents a systems-level organisation in which different compound classes interact through feedback and feed-forward loops that stabilise cellular homeostasis and enhance functional resilience under stress conditions [28,33,44].
At the core of this network, the AMPK–SIRT1–mTORC1 signalling module functions as the central metabolic rheostat that synchronises energy availability with autophagic and biosynthetic programmes. Polysaccharides and bioactive peptides enhance this metabolic sensing by activating AMPK and upregulating SIRT1–PGC-1α, which promote mitochondrial biogenesis and efficient ATP turnover. Concurrent inhibition of mTORC1 by terpenoids, fatty acids, and polyamines maintains autophagic clearance and proteostasis, forming a coordinated energy recycling loop that sustains long-term cellular viability [188,268,278]. This integrated metabolic control ensures that nutrient signals couple with redox balance and catabolic adaptation, preventing the metabolic rigidity characteristic of ageing tissues [32,189].
Redox regulation forms the second hierarchical tier of this coupling system. Activation of Nrf2 by polyphenols, terpenoids, and polysaccharides enhances transcription of antioxidant enzymes, including SOD, CAT, GPx, and HO-1, thereby reinforcing mitochondrial and cytosolic redox buffering. Simultaneously, suppression of NF-κB and MAPK signalling by peptides, fatty acids, and polyamines limits pro-inflammatory cytokine release and oxidative amplification. Reciprocal inhibition between the Nrf2 and NF-κB modules allows precise tuning of oxidative and inflammatory responses, stabilising the cellular environment and sustaining signalling fidelity across multiple tissues [187,188,274]
The third integration layer involves transcriptional and epigenetic coupling. Polyphenols, terpenoids, and polyamines remodel histone acetylation and DNA methylation via modulation of SIRT1, EP300, and TFEB, thereby linking metabolic sensing to chromatin dynamics. These epigenetic adjustments coordinate the expression of longevity-associated transcription factors such as FOXO, PPAR, and p53, ensuring balanced control of repair, apoptosis, and stem-cell renewal [48,261]. Fatty acids and peptides further contribute to this layer by modulating membrane fluidity, receptor clustering, and signal-transduction efficiency, thereby bridging transcriptional regulation with extracellular and cytoplasmic signalling continuity [216,226].
These interconnected layers operate as a dynamic adaptive network rather than isolated pathways. Metabolic sensors such as AMPK and SIRT1 integrate upstream nutrient and stress signals; redox regulators such as Nrf2 and NF-κB calibrate the antioxidant and inflammatory balance; and downstream effectors, including FOXO, mTORC1, and PPAR, translate these inputs into sustained cytoprotective gene expression. The mutual reinforcement among these axes ensures resilience against both intrinsic and extrinsic ageing stressors. When one pathway is weakened, compensatory circuits maintain energy flow, redox integrity, and proteostasis balance, achieving a form of network redundancy that underlies the durability of multi-compound interventions [30,44,185].
Collectively, these cross-connected regulatory hierarchies demonstrate that synergistic combinations of natural products function as coordinated network stabilisers rather than simple mixtures of active molecules. By engaging convergent and complementary mechanisms across metabolic, redox, and genomic dimensions, they promote cellular adaptation and longevity at both molecular and organismal levels [28,188,274].
5. Conclusions and Future Perspectives
Natural products represent a rapidly expanding frontier in anti-ageing research, bridging nutrition, pharmacology, and systems biology. Across major classes such as polysaccharides, polyphenols, peptides, fatty acids, terpenoids, and polyamines, extensive mechanistic and experimental evidence confirms their capacity to modulate oxidative stress, inflammation, mitochondrial dynamics, genomic maintenance, and autophagy regulation. Despite marked structural heterogeneity, these compounds converge upon a shared regulatory network centred on AMPK–SIRT1–mTORC1, Nrf2–ARE, NF-κB, PPAR, and FOXO cascades, collectively sustaining cellular homeostasis and longevity. Increasing evidence on combination formulations further reveals synergistic and complementary interactions that amplify these effects through integrated network pharmacology rather than single-target modulation. Nevertheless, several challenges persist. Translating preclinical discoveries into clinically validated outcomes remains constrained by variability in compound purity, bioavailability, dosage optimisation, and long-term safety evaluation. In addition, inter-individual variability in metabolic status, age-associated physiological differences, and gut microbiome composition may lead to heterogeneous responses to identical natural bioactive combinations, further complicating efficacy evaluation and translational generalisation. Moreover, uncertainties surrounding bioavailability and in vivo biotransformation remain a critical challenge, as circulating metabolites rather than parent compounds may ultimately mediate biological effects, particularly in multi-component formulations. The intrinsic complexity of multi-component interactions necessitates advanced analytical approaches such as multi-omics profiling, computational network modelling, and human-based systems biology to elucidate the precise contribution of each constituent within combination matrices. Moreover, harmonised regulatory frameworks and standardised manufacturing protocols are required to ensure product consistency and consumer safety across global markets.
Despite growing interest in multi-component natural bioactives, robust animal and clinical evidence evaluating well-defined combinations remains limited, largely due to the structural and compositional complexity of natural products. Many bioactives exist as heterogeneous mixtures in which subtle variations in derivative structures may differentially modulate the activity of relatively conserved core scaffolds, complicating mechanistic attribution and dose–effect interpretation. Source-dependent factors such as growth conditions, nutrient availability, harvest timing, and extraction procedures further introduce batch-to-batch variability, posing challenges for the qualitative and quantitative evaluation of anti-ageing efficacy. Moreover, the multi-target and network-level modes of action characteristic of natural products, while underpinning synergistic effects, may obscure causal relationships due to pathway crosstalk and regulatory feedback. Long-term clinical data assessing the safety, stability, and sustained efficacy of combined natural bioactives also remain scarce.
Looking ahead, integration of natural-product research with emerging technologies such as metabolomics, microbiome modulation, nanodelivery systems, and precision nutrition will accelerate the development of evidence-based, personalised anti-ageing interventions. In parallel, a key future direction will be the transition from empirically derived combinations to mechanism-informed, rationally designed natural bioactive formulations guided by systems-level insights. Advancing predictive frameworks that link compositional features with reproducible biological outcomes will further improve the robustness and translational reliability of multi-component anti-ageing strategies. Future investigations should prioritise translational validation through well-designed clinical trials, longitudinal biomarker monitoring, and cross-disciplinary collaboration among food scientists, clinicians, and regulatory experts. These advancements will refine the mechanistic understanding of ageing and facilitate the safe translation of natural bioactive combinations into next-generation functional foods and health-supplement therapeutics that support healthy longevity.
Statement of the Use of Generative AI and AI-Assisted Technologies in the Writing Process
AI tool was used in polishing the first draft, and then the manuscript was edited by senior authors.
Author Contributions
Conceptualization, Y.S. and J.L. (Jun Lu); Methodology/Literature search, Y.S., L.S., J.H., J.L. (Johnson Liu) and Y.F.; Literature Analysis, Y.S. and L.S.; Resources, L.S., J.L. (Johnson Liu), Y.F. and J.L. (Jun Lu); Writing—Original Draft Preparation, Y.S.; Writing—Review & Editing, Y.S., L.S., J.H., J.L. (Johnson Liu), Y.F. and J.L. (Jun Lu); Supervision, J.L. (Jun Lu).
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Funding
This research received no external funding.
Declaration of Competing Interest
Authors Yuanze Sun and Lihong Sun were employed by Fuquan Biotechnology Company Ltd. The authors declare that this company was not involved in the preparation, writing, and submission of this manuscript. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
World Health Organization. Ageing and Health. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 15 November 2025).
-
Gilbert SF. Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000; 749p. [Google Scholar]
-
Gems D. What is an anti-aging treatment? Exp. Gerontol. 2014, 58, 14–18. DOI:10.1016/j.exger.2014.07.003 [Google Scholar]
-
Ok SC. Insights into the Anti-Aging Prevention and Diagnostic Medicine and Healthcare. Diagnostics 2022, 12, 819. DOI:10.3390/diagnostics12040819 [Google Scholar]
-
Babich O, Ivanova S, Bakhtiyarova A, Kalashnikova O, Sukhikh S. Medicinal plants are the basis of natural cosmetics. Process Biochem. 2025, 154, 35–51. DOI:10.1016/j.procbio.2025.04.009 [Google Scholar]
-
Bhattacharya T, Dey PS, Akter R, Kabir MT, Rahman MH, Rauf A. Effect of natural leaf extracts as phytomedicine in curing geriatrics. Exp. Gerontol. 2021, 150, 111352. DOI:10.1016/j.exger.2021.111352 [Google Scholar]
-
Bjørklund G, Shanaida M, Lysiuk R, Butnariu M, Peana M, Sarac I, et al. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. DOI:10.3390/molecules27207084 [Google Scholar]
-
Cătană CS, Atanasov AG, Berindan-Neagoe I. Natural products with anti-aging potential: Affected targets and molecular mechanisms. Biotechnol. Adv. 2018, 36, 1649–1656. DOI:10.1016/j.biotechadv.2018.03.012 [Google Scholar]
-
Görünmek M, Ballık B, Çakmak ZE, Çakmak T. Mycosporine-like amino acids in microalgae and cyanobacteria: Biosynthesis, diversity, and applications in biotechnology. Algal Res. 2024, 80, 103507. DOI:10.1016/j.algal.2024.103507 [Google Scholar]
-
Pilz M, Cavelius P, Qoura F, Awad D, Brück T. Lipopeptides development in cosmetics and pharmaceutical applications: A comprehensive review. Biotechnol. Adv. 2023, 67, 108210. DOI:10.1016/j.biotechadv.2023.108210 [Google Scholar]
-
Vaishnav P, Demain AL. Unexpected applications of secondary metabolites. Biotechnol. Adv. 2011, 29, 223–229. DOI:10.1016/j.biotechadv.2010.11.006 [Google Scholar]
-
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. DOI:10.1016/j.cell.2013.05.039 [Google Scholar]
-
Lumen Learning. Biology of Aging. Medicine LibreTexts. 2025. Available online: https://med.libretexts.org/Bookshelves/Gerontology/Biology_of_Aging_(Lumen)/zz%3A_Back_Matter/30%3A_Detailed_Licensing (accessed on 15 November 2025).
-
Obradovic D. Five-factor theory of aging and death due to aging. Arch. Gerontol. Geriatr. 2025, 129, 105665. DOI:10.1016/j.archger.2024.105665 [Google Scholar]
-
Jin K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. PMCID: PMC2995895. [Google Scholar]
-
Trubitsyn AG. The Mechanism of Programmed Aging: The Way to Create a Real Remedy for Senescence. Curr. Aging Sci. 2020, 13, 31–41. DOI:10.2174/1874609812666191014111422 [Google Scholar]
-
Sefanacci RG. Overview of Aging. MSD MANUAL. 2025. Available online: https://www.msdmanuals.com/home/older-people-s-health-issues/the-aging-body/overview-of-aging (accessed on 15 November 2025).
-
Shokolenko IN. Aging: A mitochondrial DNA perspective, critical analysis and an update. World J. Exp. Med. 2014, 4, 46. DOI:10.5493/wjem.v4.i4.46 [Google Scholar]
-
Diggs J. The Cross-Linkage Theory of Aging. In Encyclopedia of Aging and Public Health; Loue SJ, Sajatovic M, Eds.; Springer: Boston, MA, USA, 2008; pp. 250–252. Available online: http://link.springer.com/10.1007/978-0-387-33754-8_112 (accessed on 15 November 2025).
-
Li H, Qi Y, Jasper H. Preventing Age-Related Decline of Gut Compartmentalization Limits Microbiota Dysbiosis and Extends Lifespan. Cell Host Microbe 2016, 19, 240–253. DOI:10.1016/j.chom.2016.01.008 [Google Scholar]
-
Li YR, Trush M. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9. DOI:10.20455/ros.2016.803 [Google Scholar]
-
Sohal RS. The Rate of Living Theory: A Contemporary Interpretation. In Insect Aging; Collatz KG, Sohal RS, Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 23–44. Available online: http://link.springer.com/10.1007/978-3-642-70853-4_3 (accessed on 15 November 2025).
-
Tohyama S, Fujita J, Hishiki T, Matsuura T, Hattori F, Ohno R, et al. Glutamine Oxidation Is Indispensable for Survival of Human Pluripotent Stem Cells. Cell Metab. 2016, 23, 663–674. DOI:10.1016/j.cmet.2016.03.001 [Google Scholar]
-
Anton B, Vitetta L, Cortizo F, Sali A. Can we delay aging? The biology and science of aging. Ann. New York Acad. Sci. 2006, 1057, 525–535. DOI:10.1196/annals.1356.040 [Google Scholar]
-
Da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T. A synopsis on aging—Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. DOI:10.1016/j.arr.2016.06.005 [Google Scholar]
-
Rosen RS, Yarmush ML. Current Trends in Anti-Aging Strategies. Annu. Rev. Biomed. Eng. 2023, 25, 363–385. DOI:10.1146/annurev-bioeng-120122-123054 [Google Scholar]
-
Li Y, Tian X, Luo J, Bao T, Wang S, Wu X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. DOI:10.1186/s12964-024-01663-1 [Google Scholar]
-
Fan WY, Fan XX, Xie YJ, Yan XD, Tao MX, Zhao SL, et al. Research progress on the anti-aging effects and mechanisms of polysaccharides from Chinese herbal medicine. Food Med. Homol. 2025, 3, 9420108. DOI:10.26599/FMH.2026.9420108 [Google Scholar]
-
Li J, Wang J, Zhang N, Li Y, Cai Z, Li G, et al. Anti-aging activity and their mechanisms of natural food-derived peptides: Current advancements. Food Innov. Adv. 2023, 2, 272–290. DOI:10.48130/FIA-2023-0028 [Google Scholar]
-
Teng H, Xiao H, Li X, Huang J, Zhang B, Zeng M. Recent advances in the anti-aging effects of natural polysaccharides: Sources, structural characterization, action mechanisms and structure-activity relationships. Trends Food Sci. Technol. 2025, 160, 105000. DOI:10.1016/j.tifs.2025.105000 [Google Scholar]
-
Xiong R, Zhou X, Tang Y, Wu J, Sun Y, Teng J, et al. Lychee seed polyphenol protects the blood–brain barrier through inhibiting Aβ(25–35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice. Phytother. Res. 2020, 35, 954–973. DOI:10.1002/ptr.6849 [Google Scholar]
-
Zhong R, Shen L, Fan Y, Luo Q, Hong R, Sun X, et al. Anti-aging mechanism and effect of treatment with raw and wine-steamed Polygonatum sibiricum on D-galactose-induced aging in mice by inhibiting oxidative stress and modulating gut microbiota. Front. Pharmacol. 2024, 15, 1335786. DOI:10.3389/fphar.2024.1335786 [Google Scholar]
-
Shen C, Jiang J, Yang L, Wang D, Zhu W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2016, 174, 1395–1425. DOI:10.1111/bph.13631 [Google Scholar]
-
Zhou S, Gu J, Liu R, Wei S, Wang Q, Shen H, et al. Spermine Alleviates Acute Liver Injury by Inhibiting Liver-Resident Macrophage Pro-Inflammatory Response Through ATG5-Dependent Autophagy. Front. Immunol. 2018, 9, 948. DOI:10.3389/fimmu.2018.00948 [Google Scholar]
-
Zhu W, Xiao F, Tang Y, Zou W, Li X, Zhang P, et al. Spermidine prevents high glucose-induced senescence in HT-22 cells by upregulation of CB1 receptor. Clin. Exp. Pharmacol. Physio. 2018, 45, 832–840. DOI:10.1111/1440-1681.12955 [Google Scholar]
-
Feng J, Liu S, Ma S, Zhao J, Zhang W, Qi W, et al. Protective effects of resveratrol on postmenopausal osteoporosis: Regulation of SIRT1-NF-κB signaling pathway. Acta Biochim. Et. Biophys. Sin. 2014, 46, 1024–1033. DOI:10.1093/abbs/gmu103 [Google Scholar]
-
Luo J, Si H, Jia Z, Liu D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. DOI:10.3390/antiox10020283 [Google Scholar]
-
Suzuki T, Ohishi T, Tanabe H, Miyoshi N, Nakamura Y. Anti-Inflammatory Effects of Dietary Polyphenols through Inhibitory Activity against Metalloproteinases. Molecules 2023, 28, 5426. DOI:10.3390/molecules28145426 [Google Scholar]
-
Ji J, Yan H, Ye Y, Huang Z, Wang Y, Sun J, et al. Plant polysaccharides with anti-aging effects and mechanism in evaluation model Caenorhabditis elegans. Int. J. Biol. Macromol. 2025, 308, 142268. DOI:10.1016/j.ijbiomac.2025.142268 [Google Scholar]
-
Pandey KB, Rizvi SI. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. DOI:10.4161/oxim.2.5.9498 [Google Scholar]
-
Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA. Role of protein kinase C δ in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem. Biophys. Res. Commun. 2006, 341, 1007–1016. DOI:10.1016/j.bbrc.2006.01.065 [Google Scholar]
-
Xing Q, Fei W, Xu R, Fei J. Fundamental Organic Chemistry, 3rd ed.; Higher Education Press: Beijing, China, 2005. [Google Scholar]
-
Lakey-Beitia J, Burillo AM, La Penna G, Hegde ML, Rao KS, Rao KSJ, et al. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. DOI:10.3233/JAD-200185 [Google Scholar]
-
Mu S, Yang W, Huang G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2020, 97, 628–632. DOI:10.1111/cbdd.13798 [Google Scholar]
-
Govindharaj D, Nachimuthu S, Gonsalves DF, Kothandan R, Dhurai B, Rajamani L, et al. Molecular Docking Analysis of Chlorogenic Acid Against Matrix Metalloproteinases (MMPs). Biointerface Res. Appl. Chem. 2020, 10, 6865–6873. DOI:10.33263/BRIAC106.68656873 [Google Scholar]
-
Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, De La Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J. Nutr. Biochem. 2013, 24, 1401–1413. DOI:10.1016/j.jnutbio.2012.11.008 [Google Scholar]
-
Park JJ, Lee WY. Anti-glycation effect of Ecklonia cava polysaccharides extracted by combined ultrasound and enzyme-assisted extraction. Int. J. Biol. Macromol. 2021, 180, 684–691. DOI:10.1016/j.ijbiomac.2021.03.118 [Google Scholar]
-
Proshkina E, Plyusnin S, Babak T, Lashmanova E, Maganova F, Koval L, et al. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529. DOI:10.3390/antiox9060529 [Google Scholar]
-
Shen Y, Zhao H, Wang X, Wu S, Wang Y, Wang C, et al. Unraveling the web of defense: the crucial role of polysaccharides in immunity. Front. Immunol. 2024, 15, 1406213. DOI:10.3389/fimmu.2024.1406213 [Google Scholar]
-
Gong L, Hou J, Yang H, Zhang X, Zhao J, Wang L, et al. Kuntai capsule attenuates premature ovarian insufficiency by activating the FOXO3/SIRT5 signaling pathway in mice: A comprehensive study using UHPLC-LTQ-Orbitrap and integrated pharmacology. J. Ethnopharmacol. 2024, 322, 117625. DOI:10.1016/j.jep.2023.117625 [Google Scholar]
-
Li R, Yi Q, Wang J, Miao Y, Chen Q, Xu Y, et al. Paeonol promotes longevity and fitness in Caenorhabditis elegans through activating the DAF-16/FOXO and SKN-1/Nrf2 transcription factors. Biomed. Pharmacother. 2024, 173, 116368. DOI:10.1016/j.biopha.2024.116368 [Google Scholar]
-
De Carvalho C, Caramujo M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. DOI:10.3390/molecules23102583 [Google Scholar]
-
Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. DOI:10.1194/jlr.R067629 [Google Scholar]
-
Tvrzicka E, Kremmyda LS, Stankova B, Zak A. FATTY ACIDS AS BIOCOMPOUNDS: THEIR ROLE IN HUMAN METABOLISM, HEALTH AND DISEASE—A REVIEW. PART 1: CLASSIFICATION, DIETARY SOURCES AND BIOLOGICAL FUNCTIONS. Biomed. Pap. 2011, 155, 117–130. DOI:10.5507/bp.2011.038 [Google Scholar]
-
Hussey B, Lindley MR, Mastana SS. Omega 3 fatty acids, inflammation and DNA methylation: An overview. CliniCal Lipidol. 2017, 2, 24–32. DOI:10.1080/17584299.2017.1319454 [Google Scholar]
-
Moradi S, Alivand M, KhajeBishak Y, AsghariJafarabadi M, Alipour M, Chilibeck PD, et al. The effect of omega3 fatty acid supplementation on PPARγ and UCP2 expressions, resting energy expenditure, and appetite in athletes. BMC Sports Sci. Med. Rehabil. 2021, 13, 48. DOI:10.1186/s13102-021-00266-4 [Google Scholar]
-
Xie SH, Li H, Jiang JJ, Quan Y, Zhang HY. Multi-Omics Interpretation of Anti-Aging Mechanisms for ω-3 Fatty Acids. Genes 2021, 12, 1691. DOI:10.3390/genes12111691 [Google Scholar]
-
Álvarez-Mercado AI, Plaza-Diaz J. Dietary Polysaccharides as Modulators of the Gut Microbiota Ecosystem: An Update on Their Impact on Health. Nutrients 2022, 14, 4116. DOI:10.3390/nu14194116 [Google Scholar]
-
Parkar SG, Trower TM, Stevenson DE. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. DOI:10.1016/j.anaerobe.2013.07.009 [Google Scholar]
-
Rana A, Samtiya M, Dhewa T, Mishra V, Aluko RE. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. DOI:10.1111/jfbc.14264 [Google Scholar]
-
Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, et al. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. DOI:10.3389/fphar.2022.806470 [Google Scholar]
-
Auguste S, Yan B, Guo M. Induction of mitophagy by green tea extracts and tea polyphenols: A potential anti-aging mechanism of tea. Food Biosci. 2023, 55, 102983. DOI:10.1016/j.fbio.2023.102983 [Google Scholar]
-
Chandran AK, Stach M, Kucharska AZ, Sokół-Łętowska A, Szumny A, Moreira H, et al. Comparison of polyphenol and volatile compounds and in vitro antioxidant, anti-inflammatory, antidiabetic, anti-ageing, and anticancer activities of dry tea leaves. LWT 2025, 222, 117632. DOI:10.1016/j.lwt.2025.117632 [Google Scholar]
-
Liang H, Qu M, Ang S, Li D, He C. Anti-aging effect of tea and its phytochemicals. Food Res. Int. 2025, 214, 116572. DOI:10.1016/j.foodres.2025.116572 [Google Scholar]
-
Zhang G, Jiang W, Hu Q, Luo J, Peng X. Characterization and Anti-Aging Potency of Phenolic Compounds in Xianhu Tea Extracts. Foods 2025, 14, 737. DOI:10.3390/foods14050737 [Google Scholar]
-
Cogoi L, Marrassini C, Saint Martin EM, Alonso MR, Filip R, Anesini C. Inhibition of Glycation End Products Formation and Antioxidant Activities of Ilex paraguariensis: comparative study of fruit and leaves extracts. J. Pharmacopunct. 2023, 26, 338–347. DOI:10.3831/KPI.2023.26.4.338 [Google Scholar]
-
Gao W, Zhao C, Shang X, Li B, Guo J, Wang J, et al. Ameliorative Effects of Raisin Polyphenol Extract on Oxidative Stress and Aging In Vitro and In Vivo via Regulation of Sirt1–Nrf2 Signaling Pathway. Foods 2024, 14, 71. DOI:10.3390/foods14010071 [Google Scholar]
-
Hu M, Cheng N, Liu R, Shao L, Yang R, Du S, et al. Red Ginseng Aqueous Extract Superior to White Ginseng Exhibited Anti-Aging Property Through IIS Signaling Pathway in Caenorhabditis elegans. Chem. Biodivers. 2025, 22, e202500604. DOI:10.1002/cbdv.202500604 [Google Scholar]
-
Wu M, Cai J, Fang Z, Li S, Huang Z, Tang Z, et al. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutr. 2022, 14, 857. DOI:10.3390/nu14040857 [Google Scholar]
-
Hao M, Ding C, Peng X, Chen H, Dong L, Zhang Y, et al. Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine 2022, 105, 154365. DOI:10.1016/j.phymed.2022.154365 [Google Scholar]
-
Zefzoufi M, Fdil R, Bouamama H, Gadhi C, Katakura Y, Mouzdahir A, et al. Effect of extracts and isolated compounds derived from Retama monosperma (L.) Boiss. on anti-aging gene expression in human keratinocytes and antioxidant activity. J. Ethnopharmacol. 2021, 280, 114451. DOI:10.1016/j.jep.2021.114451 [Google Scholar]
-
Li S, He Y, Zhong S, Li Y, Di Y, Wang Q, et al. Antioxidant and Anti-Aging Properties of Polyphenol–Polysaccharide Complex Extract from Hizikia fusiforme. Foods 2023, 12, 3725. DOI:10.3390/foods12203725 [Google Scholar]
-
Yin J, Liu X, Peng F, Wang Q, Xiao Y, Liu S. Metabolite profiling, antioxidant and anti-aging activities of Siraitia grosvenorii pomace processed by solid-state fermentation with Eurotium cristatum. Process Biochem. 2023, 133, 109–120. DOI:10.1016/j.procbio.2023.08.016 [Google Scholar]
-
Bucciantini M, Leri M, Scuto M, Ontario M, Trovato Salinaro A, Calabrese EJ, et al. Xenohormesis underlyes the anti-aging and healthy properties of olive polyphenols. Mech. Ageing Dev. 2022, 202, 111620. DOI:10.1016/j.mad.2022.111620 [Google Scholar]
-
Imb M, Véghelyi Z, Maurer M, Kühnel H. Exploring Senolytic and Senomorphic Properties of Medicinal Plants for Anti-Aging Therapies. Int. J. Mol. Sci. 2024, 25, 10419. DOI:10.3390/ijms251910419 [Google Scholar]
-
Qin Y, Chen F, Tang Z, Ren H, Wang Q, Shen N, et al. Ligusticum chuanxiong Hort as a medicinal and edible plant foods: Antioxidant, anti-aging and neuroprotective properties in Caenorhabditis elegans. Front. Pharmacol. 2022, 13, 1049890. DOI:10.3389/fphar.2022.1049890 [Google Scholar]
-
Xu Y, Song J, Huang Q, Wei X, Deng Z, Song Z, et al. Functional foods and nutraceuticals with anti-aging effects: Focus on modifying the enteral microbiome. J. Funct. Foods 2025, 128, 106786. DOI:10.1016/j.jff.2025.106786 [Google Scholar]
-
Bragais EKB, Medina PMB. Effects of starter cultures on the metabolite profile, antioxidant activities, and anti-aging properties of tapuy lees. Discov. Food 2025, 5, 17. DOI:10.1007/s44187-025-00285-x [Google Scholar]
-
Pusev MS, Klein OI, Gessler NN, Bachurina GP, Filippovich SY, Isakova EP, et al. Effect of Dihydroquercetin During Long-Last Growth of Yarrowia lipolytica Yeast: Anti-Aging Potential and Hormetic Properties. Int. J. Mol. Sci. 2024, 25, 12574. DOI:10.3390/ijms252312574 [Google Scholar]
-
Carvalho MJ, Pedrosa SS, Mendes A, Azevedo-Silva J, Fernandes J, Pintado M, et al. Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE). Int. J. Mol. Sci. 2023, 25, 21. DOI:10.3390/ijms25010021 [Google Scholar]
-
Coutinho TE, Martins-Gomes C, Machado-Carvalho L, Nunes FM, Silva AM. Anti-Inflammatory, Anti-Hyperglycemic, and Anti-Aging Activities of Aqueous and Methanolic Fractions Obtained from Cucurbita ficifolia Bouché Fruit Pulp and Peel Extracts. Molecules 2025, 30, 557. DOI:10.3390/molecules30030557 [Google Scholar]
-
Peng B, Hao Y, Chen Y, Yu S, Qu L. Chemical constituents and bioactivities of fermented rose (from Yunnan) extract. Nat. Prod. Res. 2025, 39, 6145–6152. DOI:10.1080/14786419.2024.2371995 [Google Scholar]
-
Auguste S, Yan B, Magina R, Xue L, Neto C, Guo M. Cranberry extracts and cranberry polyphenols induce mitophagy in human fibroblast cells. Food Biosci. 2024, 57, 103549. DOI:10.1016/j.fbio.2023.103549 [Google Scholar]
-
Tang Z, Zhao Z, Chen S, Lin W, Wang Q, Shen N, et al. Dragon fruit-kiwi fermented beverage: In vitro digestion, untargeted metabolome analysis and anti-aging activity in Caenorhabditis elegans. Front. Nutr. 2023, 9, 1052818. DOI:10.3389/fnut.2022.1052818 [Google Scholar]
-
Thilakarathna GC, Navaratne SB, Wickramasinghe I, Ranasinghe P, Samarkoon SR, Samarasekera JKRR. The effect of Salacia reticulata, Syzygium cumini, Artocarpus heterophyllus, and Cassia auriculata on controlling the rapid formation of advanced glycation end-products. J. Ayurveda Integr. Med. 2021, 12, 261–268. DOI:10.1016/j.jaim.2020.10.010 [Google Scholar]
-
Brinkmann V, Romeo M, Larigot L, Hemmers A, Tschage L, Kleinjohann J, et al. Aryl Hydrocarbon Receptor-Dependent and -Independent Pathways Mediate Curcumin Anti-Aging Effects. Antioxidants 2022, 11, 613. DOI:10.3390/antiox11040613 [Google Scholar]
-
Chen T, Luo S, Wang X, Zhou Y, Dai Y, Zhou L, et al. Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway. Antioxidants 2021, 10, 1744. DOI:10.3390/antiox10111744 [Google Scholar]
-
Zhang S, Duangjan C, Tencomnao T, Wu L, Wink M, Lin J. Oolonghomobisflavans exert neuroprotective activities in cultured neuronal cells and anti-aging effects in Caenorhabditis elegans. Front. Aging Neurosci. 2022, 14, 967316. DOI:10.3389/fnagi.2022.967316 [Google Scholar]
-
Li M, Xu X, Jia Y, Yuan Y, Na G, Zhu L, et al. Transformation of mulberry polyphenols by Lactobacillus plantarum SC-5: Increasing phenolic acids and enhancement of anti-aging effect. Food Res. Int. 2024, 192, 114778. DOI:10.1016/j.foodres.2024.114778 [Google Scholar]
-
Hemagirri M, Chen Y, Gopinath SCB, Adnan M, Patel M, Sasidharan S. RNA-sequencing exploration on SIR2 and SOD genes in Polyalthia longifolia leaf methanolic extracts (PLME) mediated anti-aging effects in Saccharomyces cerevisiae BY611 yeast cells. Biogerontology 2024, 25, 705–737. DOI:10.1007/s10522-024-10104-y [Google Scholar]
-
Abdelfattah MAO, Dmirieh M, Ben Bakrim W, Mouhtady O, Ghareeb MA, Wink M, et al. Antioxidant and anti-aging effects of Warburgia salutaris bark aqueous extract: Evidences from in silico, in vitro and in vivo studies. J. Ethnopharmacol. 2022, 292, 115187. DOI:10.1016/j.jep.2022.115187 [Google Scholar]
-
Peng X, Hao M, Zhao Y, Cai Y, Chen X, Chen H, et al. Red ginseng has stronger anti-aging effects compared to ginseng possibly due to its regulation of oxidative stress and the gut microbiota. Phytomedicine 2021, 93, 153772. DOI:10.1016/j.phymed.2021.153772 [Google Scholar]
-
Al-Harrasi A, Ali L, Ceniviva E, Al-Rawahi A, Hussain J, Hussain H, et al. Antiglycation and Antioxidant Activities and HPTLC Analysis of Boswellia sacra Oleogum Resin: The Sacred Frankincense. Trop. J. Pharm. Res. 2013, 12, 597–602. DOI:10.4314/tjpr.v12i4.23 [Google Scholar]
-
Feng Q, Li G, Xia W, Dai G, Zhou J, Xu Y, et al. The anti-aging effects of Renshen Guben on thyrotoxicosis mice: Improving immunosenescence, hypoproteinemia, lipotoxicity, and intestinal flora. Front. Immunol. 2022, 13, 983501. DOI:10.3389/fimmu.2022.983501 [Google Scholar]
-
Matacchione G, Borgonetti V, Ramini D, Silvestrini A, Ojetti M, Galeotti N, et al. Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells. Biology 2023, 12, 438. DOI:10.3390/biology12030438 [Google Scholar]
-
Wichayapreechar P, Charoenjittichai R, Prasansuklab A, Vinardell MP, Rungseevijitprapa W. Exploring the In Vitro Antioxidant, Anti-Aging, and Cytotoxic Properties of Kaempferia galanga Linn. Rhizome Extracts for Cosmeceutical Formulations. Cosmetics 2024, 11, 97. DOI:10.3390/cosmetics11030097 [Google Scholar]
-
Saad AR, Mohyeldin MM, Takla SS, Metwally AM, Ibrahim RS. Unlocking the secrets of green and roasted coffee species: identifying anti-aging compounds through UPLC-MS/MS profiling and multivariate analysis. Microchem. J. 2025, 217, 114923. DOI:10.1016/j.microc.2025.114923 [Google Scholar]
-
Kudryavtseva A, Krasnov G, Lipatova A, Alekseev B, Maganova F, Shaposhnikov M, et al. Effects of Abies sibirica terpenes on cancer- and aging-associated pathways in human cells. Oncotarget 2016, 7, 83744–83754. DOI:10.18632/oncotarget.13467 [Google Scholar]
-
Meng J, Cheng M, Liu L, Sun J, Condori-Apfata JA, Zhao D, et al. In-vitro antioxidant and in-vivo anti-aging with stress resistance on Caenorhabditis elegans of herbaceous peony stamen tea. Int. J. Food Prop. 2021, 24, 1349–1366. DOI:10.1080/10942912.2021.1967385 [Google Scholar]
-
Stavropoulou MI, Stathopoulou K, Cheilari A, Benaki D, Gardikis K, Chinou I, et al. NMR metabolic profiling of Greek propolis samples: Comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharm. Biomed. Anal. 2021, 194, 113814. DOI:10.1016/j.jpba.2020.113814 [Google Scholar]
-
Elgamal AM, Ahmed RF, Abd-ElGawad AM, El Gendy AENG, Elshamy AI, Nassar MI. Chemical Profiles, Anticancer, and Anti-Aging Activities of Essential Oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. DOI:10.3390/plants10040667 [Google Scholar]
-
Prommaban A, Kheawfu K, Chittasupho C, Sirilun S, Hemsuwimon K, Chaiyana W. Phytochemical, Antioxidant, Antihyaluronidase, Antityrosinase, and Antimicrobial Properties of Nicotiana tabacum L. Leaf Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 5761764. DOI:10.1155/2022/5761764 [Google Scholar]
-
Teratani T, Kasahara N, Ijichi T, Fujimoto Y, Sakuma Y, Sata N, et al. Activation of whole body by high levels of polyamine intake in rats. Amino Acids 2021, 53, 1695–1703. DOI:10.1007/s00726-021-03079-4 [Google Scholar]
-
Xu TT, Li H, Dai Z, Lau GK, Li BY, Zhu WL, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401–6414. DOI:10.18632/aging.103035 [Google Scholar]
-
Du ZQ, Xie JB, Ji SY, Zhou W, Tao ZS. Spermidine prevents iron overload-induced impaired bone mass by activating SIRT1/SOD2 signaling in senile rat model. Redox Rep. 2025, 30, 2485666. DOI:10.1080/13510002.2025.2485666 [Google Scholar]
-
Yang J, Zhang CR, Li ZX, Gao YH, Jiang L, Zhang J, et al. Spermine alleviates myocardial cell aging by inhibiting mitochondrial oxidative stress damage. Eur. J. Pharmacol. 2025, 997, 177477. DOI:10.1016/j.ejphar.2025.177477 [Google Scholar]
-
Kim G, Kim M, Kim M, Park C, Yoon Y, Lim DH, et al. Spermidine-induced recovery of human dermal structure and barrier function by skin microbiome. Commun. Biol. 2021, 4, 231. DOI:10.1038/s42003-020-01619-4 [Google Scholar]
-
Liu Y, Hu W, Pei X, Zhu J, Meng H, Lyu Y, et al. Polydeoxyribonucleotide-spermidine nanoparticles: Preparation, characterization, and verification of skin anti-aging efficacy. Int. J. Biol. Macromol. 2025, 322, 146984. DOI:10.1016/j.ijbiomac.2025.146984 [Google Scholar]
-
Ueno D, Ikeda K, Yamazaki E, Katayama A, Urata R, Matoba S. Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice. Sci. Rep. 2023, 13, 8338. DOI:10.1038/s41598-023-35447-3 [Google Scholar]
-
Guan B, Li C, Yang Y, Lu Y, Sun Y, Su L, et al. Effect of spermidine on radiation-induced long-term bone marrow cell injury. Int. Immunopharmacol. 2023, 114, 109557. DOI:10.1016/j.intimp.2022.109557 [Google Scholar]
-
Jeong JW, Cha HJ, Han MH, Hwang SJ, Lee DS, Yoo JS, et al. Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol. Ther. 2018, 26, 146–156. DOI:10.4062/biomolther.2016.272 [Google Scholar]
-
Kojić D, Spremo J, Đorđievski S, Čelić T, Vukašinović E, Pihler I, et al. Spermidine supplementation in honey bees: Autophagy and epigenetic modifications. PLoS ONE 2024, 19, e0306430. DOI:10.1371/journal.pone.0306430 [Google Scholar]
-
Rossmann C, Darko A, Kager G, Ledinski G, Wonisch W, Wagner T, et al. Natural Polyamine Spermidine Inhibits the In Vitro Oxidation of LDL. Molecules 2025, 30, 955. DOI:10.3390/molecules30040955 [Google Scholar]
-
Uemura T, Matsunaga M, Yokota Y, Takao K, Furuchi T. Inhibition of Polyamine Catabolism Reduces Cellular Senescence. Int. J. Mol. Sci. 2023, 24, 13397. DOI:10.3390/ijms241713397 [Google Scholar]
-
Baek AR, Hong J, Song KS, Jang AS, Kim DJ, Chin SS, et al. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020, 52, 2034–2045. DOI:10.1038/s12276-020-00545-z [Google Scholar]
-
Jiang D, Guo Y, Niu C, Long S, Jiang Y, Wang Z, et al. Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. Int. J. Mol. Sci. 2023, 24, 5793. DOI:10.3390/ijms24065793 [Google Scholar]
-
Wei C, Wang Y, Li M, Li H, Lu X, Shao H, et al. Spermine inhibits Endoplasmic Reticulum Stress—Induced Apoptosis: A New Strategy to Prevent Cardiomyocyte Apoptosis. Cell Physiol. Biochem. 2016, 38, 531–544. DOI:10.1159/000438648 [Google Scholar]
-
Bai J, Zhang Y, Li N, Cui Z, Zhang H, Liu Y, et al. Supplementation of spermidine enhances the quality of postovulatory aged porcine oocytes. Cell Commun. Signal. 2024, 22, 499. DOI:10.1186/s12964-024-01881-7 [Google Scholar]
-
Wirth A, Wolf B, Huang CK, Glage S, Hofer SJ, Bankstahl M, et al. Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. GeroScience 2021, 43, 673–690. DOI:10.1007/s11357-020-00310-0 [Google Scholar]
-
Qiang Y, Min Y, Li W, Chen D, Zhang S, Chen H, et al. Mitigating natural aging in Drosophila melanogaster with Tremella fuciformis polysaccharides. Food Biosci. 2025, 71, 107145. DOI:10.1016/j.fbio.2025.107145 [Google Scholar]
-
Finazzi M, Bovio F, Forcella M, Lasagni M, Fusi P, Di Gennaro P. Beneficial Effects of In Vitro Reconstructed Human Gut Microbiota by Ginseng Extract Fermentation on Intestinal Cell Lines. Microorganisms 2025, 13, 192. DOI:10.3390/microorganisms13010192 [Google Scholar]
-
Fang Z, Li Y, Chen H, Lian C, Zhang X, Wang J, et al. Crude polysaccharides of Helvella leucopus induces myogenic differentiation of C2C12 cells and raises levels of genes involved in mitochondrial dynamics. J. Funct. Foods 2024, 119, 106291. DOI:10.1016/j.jff.2024.106291 [Google Scholar]
-
Duan H, Li J, Fan L. Agaricus bisporus Polysaccharides Ameliorates Behavioural Deficits in D-Galactose-Induced Aging Mice: Mediated by Gut Microbiota. Foods 2023, 12, 424. DOI:10.3390/foods12020424 [Google Scholar]
-
Saad S, Abd-Alla H, Aly H, Shalaby N, Afify AEM, Helal H, et al. Citharexylum Spinosum: LC-ESI-TOF-MS Analysis and Anti-Aging Evolution on D-Galactose-Induced Aging through Anti-Inflammatory, Antioxidant Activity and Regulation of the Gut Microbiota in Rats. Egypt. J. Chem. 2024, 67, 527–544. DOI:10.21608/ejchem.2024.277822.9483 [Google Scholar]
-
Wei Y, Jiang Y, Liu M, Zhang X, Guo S, Su S, et al. Chemical structures of Lycii fructus polysaccharides tailored the gut microbiota composition of aged Caenorhabditis elegans. Food Hydrocoll. 2025, 169, 111608. DOI:10.1016/j.foodhyd.2025.111608 [Google Scholar]
-
Fang Z, Liu N, Fan Z, Wang W, Luo J, Qiu Y, et al. Cistanche deserticola polysaccharides—Companilactobacillus crustorum LBK 1001 complex relieves aging in Caenorhabditis elegans. Food Biosci. 2025, 68, 106486. DOI:10.1016/j.fbio.2025.106486 [Google Scholar]
-
Feng ST, You CT, Pan ZK, Gao WY, Wang DD, Hu LL. The anti-aging effects of Chlorella polysaccharide extract in Caenorhabditis elegans. Nat. Prod. Res. 2025, 39, 6390–6395. DOI:10.1080/14786419.2024.2371562 [Google Scholar]
-
Feng A, Zhao Z, Liu C, Du C, Gao P, Liu X, et al. Study on characterization of Bupleurum chinense polysaccharides with antioxidant mechanisms focus on ROS relative signaling pathways and anti-aging evaluation in vivo model. Int. J. Biol. Macromol. 2024, 266, 131171. DOI:10.1016/j.ijbiomac.2024.131171 [Google Scholar]
-
Yan X, Miao J, Zhang B, Liu H, Ma H, Sun Y, et al. Study on semi-bionic extraction of Astragalus polysaccharide and its anti-aging activity in vivo. Front. Nutr. 2023, 10, 1201919. DOI:10.3389/fnut.2023.1201919 [Google Scholar]
-
Hu Y, Zhou J, Cao Y, Zhang J, Zou L. Anti-aging effects of polysaccharides from quinoa (Chenopodium quinoa Willd.) in improving memory and cognitive function. J. Funct. Foods 2022, 94, 105097. DOI:10.1016/j.jff.2022.105097 [Google Scholar]
-
Huang Y, He M, Zhang J, Cheng S, Cheng X, Chen H, et al. White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster. Foods 2024, 13, 4034. DOI:10.3390/foods13244034 [Google Scholar]
-
Liang L, Yue Y, Zhong L, Liang Y, Shi R, Luo R, et al. Anti-aging activities of Rehmannia glutinosa Libosch. crude polysaccharide in Caenorhabditis elegans based on gut microbiota and metabonomic analysis. Int. J. Biol. Macromol. 2023, 253, 127647. DOI:10.1016/j.ijbiomac.2023.127647 [Google Scholar]
-
Luo D, Liu X, Guan J, Jang G, Hua Y, Zhang X, et al. Effects of Tremella fuciformis-Derived Polysaccharides with Different Molecular Weight on D-Galactose-Induced Aging of Mice. Pol. J. Food Nutr. Sci. 2023, 73, 163–174. DOI:10.31883/pjfns/163612 [Google Scholar]
-
Chen Y, Ouyang Y, Chen X, Chen R, Ruan Q, Farag MA, et al. Hypoglycaemic and anti-ageing activities of green alga Ulva lactuca polysaccharide via gut microbiota in ageing-associated diabetic mice. Int. J. Biol. Macromol. 2022, 212, 97–110. DOI:10.1016/j.ijbiomac.2022.05.109 [Google Scholar]
-
Yang JJ, Zhang X, Dai JF, Ma YG, Jiang JG. Effect of fermentation modification on the physicochemical characteristics and anti-aging related activities of Polygonatum kingianum polysaccharides. Int. J. Biol. Macromol. 2023, 235, 123661. DOI:10.1016/j.ijbiomac.2023.123661 [Google Scholar]
-
Bai X, Wang M, Xu T, Zhou S, Chu W. Antioxidant and anti-aging activities of Acanthopanax senticosus polysaccharide CQ-1 in nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2025, 297, 139925. DOI:10.1016/j.ijbiomac.2025.139925 [Google Scholar]
-
Okoro NO, Odiba AS, Yu Q, He B, Liao G, Jin C, et al. Polysaccharides Extracted from Dendrobium officinale Grown in Different Environments Elicit Varying Health Benefits in Caenorhabditis elegans. Nutrients 2023, 15, 2641. DOI:10.3390/nu15122641 [Google Scholar]
-
Yue Y, Liang L, Zhang H, Li C, Zhao M, Yang M, et al. Antioxidant and anti-aging effects of purified Rehmannia glutinosa polysaccharide in Caenorhabditis elegans. Process Biochem. 2024, 137, 41–53. DOI:10.1016/j.procbio.2023.12.009 [Google Scholar]
-
Zhang C, Song X, Cui W, Yang Q. Antioxidant and anti-ageing effects of enzymatic polysaccharide from Pleurotus eryngii residue. Int. J. Biol. Macromol. 2021, 173, 341–350. DOI:10.1016/j.ijbiomac.2021.01.030 [Google Scholar]
-
Cheng Y, He S, Wei Z, Meng Y, Su D, Li X, et al. Structural characterizations and anti-aging activities of a homogeneous polysaccharide derived from Coix seed-lactic acid bacteria fermentation. Int. J. Biol. Macromol. 2025, 330, 147879. DOI:10.1016/j.ijbiomac.2025.147879 [Google Scholar]
-
Wen J, Liu J, Nie Y, Wang P, Shao Y, Zhang Y, et al. A novel yolk-shell nanoparticles delivery system based on Polygonatum polysaccharides and PCC1 for synergistic anti-aging. Food Res. Int. 2025, 220, 117188. DOI:10.1016/j.foodres.2025.117188 [Google Scholar]
-
Wu L, Liu X, Hu R, Chen Y, Xiao M, Liu B, et al. Prebiotic Agrocybe cylindracea crude polysaccharides combined with Lactobacillus rhamnosus GG postpone aging-related oxidative stress in mice. Food Funct. 2022, 13, 1218–1231. DOI:10.1039/D1FO02079J [Google Scholar]
-
Guo X, Xin Q, Wei P, Hua Y, Zhang Y, Su Z, et al. Antioxidant and anti-aging activities of Longan crude and purified polysaccharide (LP-A) in nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 267, 131634. DOI:10.1016/j.ijbiomac.2024.131634 [Google Scholar]
-
Zhang X, Liu Y, Zhang X, Liu H, Wang B, Li C, et al. Structural characterization and anti-ageing activity of polysaccharide from Exocarpium Citrulli. J. Mol. Struct. 2024, 1316, 139073. DOI:10.1016/j.molstruc.2024.139073 [Google Scholar]
-
Hou M, Li X, Li X, Zhang J, Jia L. Structure Analysis, Antioxidant Activity, Anti-Aging, and Organ Protective Effects of Pholiota adiposa Residue Polysaccharides. Starch-Stärke 2023, 75, 2200237. DOI:10.1002/star.202200237 [Google Scholar]
-
Zhang X, Liu L, Luo J, Peng X. Anti-aging potency correlates with metabolites from in vitro fermentation of edible fungal polysaccharides using human fecal intestinal microflora. Food Funct. 2022, 13, 11592–11603. DOI:10.1039/D2FO01951E [Google Scholar]
-
Li Y, Zhao X, Wang J, Yu Q, Ren J, Jiang Z, et al. Characterization and anti-aging activities of polysaccharide from Rana dybowskii Guenther. Front. Pharmacol. 2024, 15, 1370631. DOI:10.3389/fphar.2024.1370631 [Google Scholar]
-
Wang W, Li S, Zhu Y, Zhu R, Du X, Cui X, et al. Effect of Different Edible Trichosanthes Germplasm on Its Seed Oil to Enhance Antioxidant and Anti-Aging Activity in Caenorhabditis elegans. Foods 2024, 13, 503. DOI:10.3390/foods13030503 [Google Scholar]
-
Mora I, Pérez-Santamaria A, Tortajada-Pérez J, Vázquez-Manrique RP, Arola L, Puiggròs F. Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. elegans. Cells 2023, 12, 1932. DOI:10.3390/cells12151932 [Google Scholar]
-
Huangfu J, Liu J, Peng C, Suen YL, Wang M, Jiang Y, et al. DHA-rich marine microalga Schizochytrium mangrovei possesses anti-ageing effects on Drosophila melanogaster. J. Funct. Foods. 2013, 5, 888–896. DOI:10.1016/j.jff.2013.01.038 [Google Scholar]
-
Ramirez-Tortosa CL, Varela-López A, Navarro-Hortal MD, Ramos-Pleguezuelos FM, Márquez-Lobo B, Ramirez-Tortosa Mc, et al. Longevity and Cause of Death in Male Wistar Rats Fed Lifelong Diets Based on Virgin Olive Oil, Sunflower Oil, or Fish Oil. J. Gerontol. Ser. A 2020, 75, 442–451. DOI:10.1093/gerona/glz091 [Google Scholar]
-
Chen J, Wu S, Wu Y, Zhuang P, Zhang Y, Jiao J. Long-term dietary DHA intervention prevents telomere attrition and lipid disturbance in telomerase-deficient male mice. Eur. J. Nutr. 2023, 62, 1867–1878. DOI:10.1007/s00394-023-03120-0 [Google Scholar]
-
Wu D, Jia Y, Liu Y, Shang M. Dose–response relationship of dietary Omega-3 fatty acids on slowing phenotypic age acceleration: A cross-sectional study. Front. Nutr. 2024, 11, 1424156. DOI:10.3389/fnut.2024.1424156 [Google Scholar]
-
González-Hedström D, Amor S, De La Fuente-Fernández M, Tejera-Muñoz A, Priego T, Martín AI, et al. A Mixture of Algae and Extra Virgin Olive Oils Attenuates the Cardiometabolic Alterations Associated with Aging in Male Wistar Rats. Antioxidants 2020, 9, 483. DOI:10.3390/antiox9060483 [Google Scholar]
-
Lu H, Li L, Zou Z, Han B, Gong M. The Therapeutic Potential of Hemp Seed Oil in D-Galactose-Induced Aging Rat Model Was Determined through the Combined Assessment of 1H NMR Metabolomics and 16S rRNA Gene Sequencing. Metabolites 2024, 14, 304. DOI:10.3390/metabo14060304 [Google Scholar]
-
Prommaban A, Kuanchoom R, Seepuan N, Chaiyana W. Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction. Plants 2021, 10, 1582. DOI:10.3390/plants10081582 [Google Scholar]
-
Vu DC, Vu QT, Huynh L, Lin CH, Alvarez S, Vo XT, et al. Evaluation of fatty acids, phenolics and bioactivities of spent coffee grounds prepared from Vietnamese coffee. Int. J. Food Prop. 2021, 24, 1548–1558. DOI:10.1080/10942912.2021.1977657 [Google Scholar]
-
Lee JH, Seo EY, Lee YM. Comparative investigation on variations of nutritional components in whole seeds and seed coats of Korean black soybeans for different crop years and screening of their antioxidant and anti-aging properties. Food Chem. X 2023, 17, 100572. DOI:10.1016/j.fochx.2023.100572 [Google Scholar]
-
Shi H, Hu X, Zheng H, Li C, Sun L, Guo Z, et al. Two novel antioxidant peptides derived from Arca subcrenata against oxidative stress and extend lifespan in Caenorhabditis elegans. J. Funct. Foods 2021, 81, 104462. DOI:10.1016/j.jff.2021.104462 [Google Scholar]
-
Ye X, Yang R, Riaz T, Chen J. Stability and antioxidant function of Porphyra haitanensis proteins during simulated gastrointestinal digestion: Effects on stress resistance and lifespan extension in Caenorhabditis elegans. Int. J. Biol. Macromol. 2025, 293, 139291. DOI:10.1016/j.ijbiomac.2024.139291 [Google Scholar]
-
He W, Xie J, Xia Z, Chen X, Xiao J, Cao Y, et al. A novel peptide derived from Haematococcus pluvialis residue exhibits anti-aging activity in Caenorhabditis elegans via the insulin/IGF-1 signaling pathway. Food Funct. 2023, 14, 5576–5588. DOI:10.1039/D3FO00383C [Google Scholar]
-
Liu J, Zhao Y, Leng F, Xiao X, Jiang W, Guo S. A Hydrolyzed Soybean Protein Enhances Oxidative Stress Resistance in C. elegans and Modulates Gut–Immune Axis in BALB/c Mice. Antioxidants 2025, 14, 689. DOI:10.3390/antiox14060689 [Google Scholar]
-
Amakye WK, Hou C, Xie L, Lin X, Gou N, Yuan E, et al. Bioactive anti-aging agents and the identification of new anti-oxidant soybean peptides. Food Biosci. 2021, 42, 101194. DOI:10.1016/j.fbio.2021.101194 [Google Scholar]
-
Sakhenberg E, Linkova N, Kraskovskaya N, Krieger D, Polyakova V, Medvedev D, et al. The Influence of Short Peptides on Cell Senescence and Neuronal Differentiation. Curr. Issues Mol. Biol. 2025, 47, 739. DOI:10.3390/cimb47090739 [Google Scholar]
-
Alnadari F, Wang R, Cheng G, Nasiru M, Baldi S, Almogahed M, et al. Development Characterization and Anti-Aging Potential of Fish Collagen Peptide-Based Beverages with Bovine Colostrum. Iran. J. Chem. Chem. Eng. 2025, 44, 1161–1176. DOI:10.30492/ijcce.2025.2037572.6729 [Google Scholar]
-
Cardeira M, Bernardo A, Leonardo IC, Gaspar FB, Marques M, Melgosa R, et al. Cosmeceutical Potential of Extracts Derived from Fishery Industry Residues: Sardine Wastes and Codfish Frames. Antioxidants 2022, 11, 1925. DOI:10.3390/antiox11101925 [Google Scholar]
-
Guo HX, Wang BB, Wu HY, Feng HY, Zhang HY, Gao W, et al. Turtle peptide and its derivative peptide ameliorated DSS-induced ulcerative colitis by inhibiting inflammation and modulating the composition of the gut microbiota. Int. Immunopharmacol. 2024, 132, 112024. DOI:10.1016/j.intimp.2024.112024 [Google Scholar]
-
Gensous N, Garagnani P, Santoro A, Giuliani C, Ostan R, Fabbri C, et al. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. GeroScience 2020, 42, 687–701. DOI:10.1007/s11357-019-00149-0 [Google Scholar]
-
Hou JY, Xu H, Cao GZ, Tian LL, Wang LH, Zhu NQ, et al. Multi-omics reveals Dengzhan Shengmai formulation ameliorates cognitive impairments in D-galactose-induced aging mouse model by regulating CXCL12/CXCR4 and gut microbiota. Front. Pharmacol. 2023, 14, 1175970. DOI:10.3389/fphar.2023.1175970 [Google Scholar]
-
Rinott E, Meir AY, Tsaban G, Zelicha H, Kaplan A, Knights D, et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. DOI:10.1186/s13073-022-01015-z [Google Scholar]
-
Yaskolka Meir A, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. DOI:10.1136/gutjnl-2020-323106 [Google Scholar]
-
Hoffmann A, Meir AY, Hagemann T, Czechowski P, Müller L, Engelmann B, et al. A polyphenol-rich green Mediterranean diet enhances epigenetic regulatory potential: The DIRECT PLUS randomized controlled trial. Metabolism 2023, 145, 155594. DOI:10.1016/j.metabol.2023.155594 [Google Scholar]
-
Zhang SJ, Xu TT, Li L, Xu YM, Qu ZL, Wang XC, et al. Bushen-Yizhi formula ameliorates cognitive dysfunction through SIRT1/ER stress pathway in SAMP8 mice. Oncotarget 2017, 8, 49338–49350. DOI:10.18632/oncotarget.17638 [Google Scholar]
-
Chen W, Liu S, Xu G, Liu X, Shi Y, Wang G, et al. Zuogui and Yougui pills extend lifespan and improve ageing biomarkers via kaempferol-mediated mitophagy in Caenorhabditis elegans. Biogerontology 2025, 26, 172. DOI:10.1007/s10522-025-10317-9 [Google Scholar]
-
Meng F, Li J, Rao Y, Wang W, Fu Y. Gengnianchun Extends the Lifespan of Caenorhabditis elegans via the Insulin/IGF-1 Signalling Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 4740739. DOI:10.1155/2018/4740739 [Google Scholar]
-
Hou L, Jiang M, Guo Q, Shi W. Zymolytic Grain Extract (ZGE) Significantly Extends the Lifespan and Enhances the Environmental Stress Resistance of Caenorhabditis elegans. Int. J. Mol. Sci. 2019, 20, 3489. DOI:10.3390/ijms20143489 [Google Scholar]
-
Wan F, Zhi D, Liu D, Xian J, Li M, AbuLizi A, et al. Lifespan extension in Caenorhabiditis elegans by several traditional Chinese medicine formulas. Biogerontology 2014, 15, 377–387. DOI:10.1007/s10522-014-9508-1 [Google Scholar]
-
Nurkolis F, Purnomo AF, Alisaputra D, Gunawan WB, Qhabibi FR, Park W, et al. In silico and in vitro studies reveal a synergistic potential source of novel anti-ageing from two Indonesian green algae. J. Funct. Foods 2023, 104, 105555. DOI:10.1016/j.jff.2023.105555 [Google Scholar]
-
Chen W, Wang J, Shi J, Yang X, Yang P, Wang N, et al. Longevity Effect of Liuwei Dihuang in Both Caenorhabditis elegans and Aged Mice. Aging Dis. 2019, 10, 578. DOI:10.14336/AD.2018.0604 [Google Scholar]
-
Zeng L, Yang Z, Yun T, Fan S, Pei Z, Chen Z, et al. Antiaging effect of a Jianpi-yangwei formula in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 313. DOI:10.1186/s12906-019-2704-4 [Google Scholar]
-
Jensen JB, Dollerup OL, Møller AB, Billeskov TB, Dalbram E, Chubanava S, et al. A randomized placebo-controlled trial of nicotinamide riboside and pterostilbene supplementation in experimental muscle injury in elderly individuals. JCI Insight 2022, 7, e158314. DOI:10.1172/jci.insight.158314 [Google Scholar]
-
Omori M. Anti-Ageing Medicine as Edible Health Insurance: Self-Care and Ageing Well among Older Adults Living in Australia and Japan. Ph.D. Dissertation, Swinburne University of Technology, Melbourne, Australia, 2015. [Google Scholar]
-
Boonpisuttinant K, Unkeaw S, Chomphoo W, Udompong S, Khong HY. In Vitro Anti-ageing Activities of the Extracts of Low-Grade Pineapple and Lime Key from Sob Prab Cooperative Limited, Lampang, Thailand. J. Smart Sci. Technol. 2022, 2, 46–59. DOI:10.24191/jsst.v2i1.24 [Google Scholar]
-
Su S, Chen G, Gao M, Zhong G, Zhang Z, Wei D, et al. Kai-Xin-San protects against mitochondrial dysfunction in Alzheimer’s disease through SIRT3/NLRP3 pathway. Chin. Med. 2023, 18, 26. DOI:10.1186/s13020-023-00722-y [Google Scholar]
-
Xing C, Zeng Z, Shan Y, Guo W, Shah R, Wang L, et al. A Network Pharmacology-based Study on the Anti-aging Properties of Traditional Chinese Medicine Sisheng Bulao Elixir. Comb. Chem. High. Throughput Screen. 2024, 27, 1840–1849. DOI:10.2174/0113862073276253231114063813 [Google Scholar]
-
Sun W, Shahrajabian MH, Cheng Q. Therapeutic Roles of Goji Berry and Ginseng in Traditional Chinese. J. Nutr. Food Secur. 2019, 4, 4293–4305. DOI:10.18502/jnfs.v4i4.1727 [Google Scholar]
-
Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. DOI:10.3390/ijms23031445 [Google Scholar]
-
Izadi M, Sadri N, Abdi A, Zadeh MMR, Jalaei D, Ghazimoradi MM, et al. Anti-ageing natural supplements: The main players in promoting healthy lifespan. Nutr. Res. Rev. 2024, 38, 522–539. DOI:10.1017/S0954422424000301 [Google Scholar]
-
La Manna S, Di Natale C, Florio D, Marasco D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. DOI:10.3390/ijms19092714 [Google Scholar]
-
Bahrami SA, Bakhtiari N. Ursolic acid regulates aging process through enhancing of metabolic sensor proteins level. Biomed. Pharmacother. 2016, 82, 8–14. DOI:10.1016/j.biopha.2016.04.047 [Google Scholar]
-
Nie H, Wang Y, Qin Y, Gong X. Oleanolic acid induces autophagic death in human gastric cancer cells in vitro and in vivo. Cell Biol. Int. 2016, 40, 770–778. DOI:10.1002/cbin.10612 [Google Scholar]
-
Song L, Zhang S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. DOI:10.3390/biom13111600 [Google Scholar]
-
Lin X, Liu W, Hu X, Liu Z, Wang F, Wang J. The role of polyphenols in modulating mitophagy: Implications for therapeutic interventions. Pharmacol. Res. 2024, 207, 107324. DOI:10.1016/j.phrs.2024.107324 [Google Scholar]
-
Safer AM, Afzal M, Nomani M, Mousa SA. Green Tea Extract in the Management of Hepatic Fibrosis. In Tea in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2013; pp. 903–909. DOI:10.1016/B978-0-12-384937-3.00076-8 [Google Scholar]
-
Zhang H, Wang M, Xiao J. Stability of polyphenols in food processing. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2022; pp. 1–45. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1043452622000158 (accessed on 15 November 2025).
-
Andrés CMC, Pérez De La Lastra JM, Juan CA, Plou FJ, Pérez-Lebeña E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. DOI:10.3390/pr11092771 [Google Scholar]
-
Wu J, Hsieh TC, Lu X, Wang Z. Induction of Quinone Reductase NQO1 by Resveratrol in Human K562 Cells Involves the Antioxidant Response Element ARE and is Accompanied by Nuclear Translocation of Transcription Factor Nrf2. Med. Chem. 2006, 2, 275–285. DOI:10.2174/157340606776930709 [Google Scholar]
-
Milenkovic D, Jude B, Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic. Biol. Med. 2013, 64, 40–51. DOI:10.1016/j.freeradbiomed.2013.05.046 [Google Scholar]
-
Quan Y, Park W, Jin J, Kim W, Park SK, Kang KP. Sirtuin 3 Activation by Honokiol Decreases Unilateral Ureteral Obstruction-Induced Renal Inflammation and Fibrosis via Regulation of Mitochondrial Dynamics and the Renal NF-κB-TGF-β1/Smad Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 402. DOI:10.3390/ijms21020402 [Google Scholar]
-
Guan Y, Li L, Yang R, Lu Y, Tang J. Targeting mitochondria with natural polyphenols for treating Neurodegenerative Diseases: A comprehensive scoping review from oxidative stress perspective. J. Transl. Med. 2025, 23, 572. DOI:10.1186/s12967-025-06605-0 [Google Scholar]
-
Mthembu SXH, Dludla PV, Ziqubu K, Nyambuya TM, Kappo AP, Madoroba E, et al. The Potential Role of Polyphenols in Modulating Mitochondrial Bioenergetics within the Skeletal Muscle: A Systematic Review of Preclinical Models. Molecules 2021, 26, 2791. DOI:10.3390/molecules26092791 [Google Scholar]
-
Stankovic S, Mutavdzin Krneta S, Djuric D, Milosevic V, Milenkovic D. Plant Polyphenols as Heart’s Best Friends: From Health Properties, to Cellular Effects, to Molecular Mechanisms of Action. Int. J. Mol. Sci. 2025, 26, 915. DOI:10.3390/ijms26030915 [Google Scholar]
-
Wu M, Luo Q, Nie R, Yang X, Tang Z, Chen H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 2175–2193. DOI:10.1080/10408398.2020.1773390 [Google Scholar]
-
Feng G, Han K, Yang Q, Feng W, Guo J, Wang J, et al. Interaction of Pyrogallol-Containing Polyphenols with Mucin Reinforces Intestinal Mucus Barrier Properties. J. Agric. Food Chem. 2022, 70, 9536–9546. DOI:10.1021/acs.jafc.2c03564 [Google Scholar]
-
Han P, Yu Y, Zhang L, Ruan Z. Citrus peel ameliorates mucus barrier damage in HFD-fed mice. J. Nutr. Biochem. 2023, 112, 109206. DOI:10.1016/j.jnutbio.2022.109206 [Google Scholar]
-
Graßmann J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. DOI:10.1016/S0083-6729(05)72015-X [Google Scholar]
-
Masyita A, Mustika Sari R, Dwi Astuti A, Yasir B, Rahma Rumata N, Emran TB, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. DOI:10.1016/j.fochx.2022.100217 [Google Scholar]
-
Rajasekar J, Perumal MK, Vallikannan B. A critical review on anti-angiogenic property of phytochemicals. J. Nutr. Biochem. 2019, 71, 1–15. DOI:10.1016/j.jnutbio.2019.04.006 [Google Scholar]
-
Everett SA, Dennis MF, Patel KB, Maddix S, Kundu SC, Willson RL. Scavenging of Nitrogen Dioxide, Thiyl, and Sulfonyl Free Radicals by the Nutritional Antioxidant 2-Carotene. J. Biol. Chem. 1996, 271, 3988–3994. DOI:10.1074/jbc.271.8.3988 [Google Scholar]
-
Okoro NO, Odiba AS, Osadebe PO, Omeje EO, Liao G, Fang W, et al. Bioactive Phytochemicals with Anti-Aging and Lifespan Extending Potentials in Caenorhabditis elegans. Molecules 2021, 26, 7323. DOI:10.3390/molecules26237323 [Google Scholar]
-
Chen L, Yao H, Chen X, Wang Z, Xiang Y, Xia J, et al. Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by d-Galactose. Neurochem. Res. 2017, 43, 430–440. DOI:10.1007/s11064-017-2438-y [Google Scholar]
-
Park YM, Park SN. Inhibitory Effect of Lupeol on MMPs Expression using Aged Fibroblast through Repeated UVA Irradiation. Photochem. Photobiol. 2018, 95, 587–594. DOI:10.1111/php.13022 [Google Scholar]
-
Hwang YP, Jeong HG. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008, 582, 2655–2662. DOI:10.1016/j.febslet.2008.06.045 [Google Scholar]
-
Wang Q, Zhao X, Jiang Y, Jin B, Wang L. Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants. Biomolecules 2023, 13, 1725. DOI:10.3390/biom13121725 [Google Scholar]
-
Lenis YY, Elmetwally MA, Maldonado-Estrada JG, Bazer FW. Physiological importance of polyamines. Zygote 2017, 25, 244–255. DOI:10.1017/S0967199417000120 [Google Scholar]
-
Sagar NA, Tarafdar S, Agarwal S, Tarafdar A, Sharma S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. DOI:10.3390/medsci9020044 [Google Scholar]
-
Michael AJ. Polyamines in Eukaryotes, Bacteria, and Archaea. J. Biol. Chem. 2016, 291, 14896–14903. DOI:10.1074/jbc.R116.734780 [Google Scholar]
-
Sarvananda L, Madhuranga D, Palihaderu PADS, Sri Lanka B. Health Effects of Polyamines: An Overview of Polyamines as a Health-Promoting Agent for Human Health. Biochem. Anal. Biochem. 2023, 12, 503. DOI:10.35248/2161-1009.23.12.503 [Google Scholar]
-
Tofalo R, Cocchi S, Suzzi G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. DOI:10.3389/fnut.2019.00016 [Google Scholar]
-
Xuan M, Gu X, Li J, Huang D, Xue C, He Y. Polyamines: Their significance for maintaining health and contributing to diseases. Cell Commun. Signal. 2023, 21, 348. DOI:10.1186/s12964-023-01373-0 [Google Scholar]
-
Arthur R, Jamwal S, Kumar P. A review on polyamines as promising next-generation neuroprotective and anti-aging therapy. Eur. J. Pharmacol. 2024, 978, 176804. DOI:10.1016/j.ejphar.2024.176804 [Google Scholar]
-
Toro-Funes N, Bosch-Fusté J, Veciana-Nogués MT, Izquierdo-Pulido M, Vidal-Carou MC. In vitro antioxidant activity of dietary polyamines. Food Res. Int. 2013, 51, 141–147. DOI:10.1016/j.foodres.2012.11.036 [Google Scholar]
-
Soda K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells 2022, 11, 164. DOI:10.3390/cells11010164 [Google Scholar]
-
Løvaas E. Antioxidative effects of polyamines. J. Am. Oil Chem. Soc. 1991, 68, 353–358. DOI:10.1007/BF02663749 [Google Scholar]
-
Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. DOI:10.1038/ncb1975 [Google Scholar]
-
Ni YQ, Liu YS. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis. 2021, 12, 1948. DOI:10.14336/AD.2021.0603 [Google Scholar]
-
Pignatti C, Tantini B, Stefanelli C, Flamigni F. Signal transduction pathways linking polyamines to apoptosis. Amino Acids 2004, 27, 359–365. DOI:10.1007/s00726-004-0115-3 [Google Scholar]
-
Seiler N, Raul F. Polyamines and apoptosis. J. Cell. Mol. Med. 2005, 9, 623–642. DOI:10.1111/j.1582-4934.2005.tb00493.x [Google Scholar]
-
Choi Y, Park H. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 2012, 19, 31. DOI:10.1186/1423-0127-19-31 [Google Scholar]
-
Hussain T, Tan B, Ren W, Rahu N, Dad R, Kalhoro DH, et al. Polyamines: Therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids 2017, 49, 1457–1468. DOI:10.1007/s00726-017-2447-9 [Google Scholar]
-
Soda K. Biological Effects of Polyamines on the Prevention of Aging-associated Diseases and on Lifespan Extension. Food Sci. Technol. Res. 2015, 21, 145–157. DOI:10.3136/fstr.21.145 [Google Scholar]
-
Zhang C, Zhen Y, Weng Y, Lin J, Xu X, Ma J, et al. Research progress on the microbial metabolism and transport of polyamines and their roles in animal gut homeostasis. J. Anim. Sci. Biotechnol. 2025, 16, 57. DOI:10.1186/s40104-025-01193-x [Google Scholar]
-
Kurihara S. Polyamine metabolism and transport in gut microbes. Biosci. Biotechnol. Biochem. 2022, 86, 957–966. DOI:10.1093/bbb/zbac080 [Google Scholar]
-
Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, et al. Microbiome–metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol. Life Sci. 2019, 76, 4961–4978. DOI:10.1007/s00018-019-03155-9 [Google Scholar]
-
Zhao Q, Huang JF, Cheng Y, Dai MY, Zhu WF, Yang XW, et al. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome 2021, 9, 224. DOI:10.1186/s40168-021-01157-z [Google Scholar]
-
Devi N, Sarmah M, Khatun B, Maji TK. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid. Interface Sci. 2017, 239, 136–145. DOI:10.1016/j.cis.2016.05.009 [Google Scholar]
-
Mizrahy S, Peer D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. DOI:10.1039/C1CS15239D [Google Scholar]
-
D’Ayala GG, Malinconico M, Laurienzo P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. DOI:10.3390/molecules13092069 [Google Scholar]
-
Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. DOI:10.1016/j.addr.2008.09.001 [Google Scholar]
-
Ngwuluka NC. Responsive polysaccharides and polysaccharides-based nanoparticles for drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Volume 1; Woodhead Publishing: Cambridge, UK, 2018; pp. 531–554. DOI:10.1016/B978-0-08-101997-9.00023-0 [Google Scholar]
-
Yang L, Zhang LM. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. DOI:10.1016/j.carbpol.2008.12.015 [Google Scholar]
-
Wang XH, Cheng XD, Wang D, Wu Z, Chen Y, Wu QX. Antioxidant and anti-aging effects of polysaccharide LDP-1 from wild Lactarius deliciosus on Caenorhabditis elegans. Food Nutr. Res. 2022, 66, 8110. DOI:10.29219/fnr.v66.8110 [Google Scholar]
-
Guo X, Luo J, Qi J, Zhao X, An P, Luo Y, et al. The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients 2022, 14, 5330. DOI:10.3390/nu14245330 [Google Scholar]
-
Wu L, Huang D, Wang S, Wang J, Guo Q, Chen X. Role and mechanism of seaweed polysaccharides in regulating mitochondrial dysfunction: A review. J. Food Sci. 2025, 90, e70100. DOI:10.1111/1750-3841.70100 [Google Scholar]
-
Ma C, Yang K, Wang Y, Dai X. Anti-Aging Effect of Agar Oligosaccharide on Male Drosophila melanogaster and Its Preliminary Mechanism. Mar. Drugs 2019, 17, 632. DOI:10.3390/md17110632 [Google Scholar]
-
Yin Z, Liang Z, Li C, Wang J, Ma C, Kang W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. DOI:10.1016/j.fshw.2021.04.001 [Google Scholar]
-
Zhang D, Liu J, Cheng H, Wang H, Tan Y, Feng W, et al. Interactions between polysaccharides and gut microbiota: A metabolomic and microbial review. Food Res. Int. 2022, 160, 111653. DOI:10.1016/j.foodres.2022.111653 [Google Scholar]
-
Lin J, Yu J, Wang X, Shi R, Liang Y, Li J, et al. Research progress on the anti-aging effect of polysaccharides of traditional Chinese medicine: Using Caenorhabditis elegans as an animal model. FASEB J. 2025, 39, e70454. DOI:10.1096/fj.202403250RR [Google Scholar]
-
Yu G, Zhang Q, Wang Y, Yang Q, Yu H, Li H, et al. Sulfated polysaccharides from red seaweed Gelidium amansii: Structural characteristics, anti-oxidant and anti-glycation properties, and development of bioactive films. Food Hydrocoll. 2021, 119, 106820. DOI:10.1016/j.foodhyd.2021.106820 [Google Scholar]
-
Li C, Dong S, Zeng A, Phyo SH, Li L, Zhao W. Structural analysis and anti-glycation activity of a novel polysaccharide isolated from Dendrobium officinale. Food Biosci. 2025, 68, 106817. DOI:10.1016/j.fbio.2025.106817 [Google Scholar]
-
Pan LH, Feng BJ, Wang JH, Zha XQ, Luo JP. Structural Characterization and Anti-glycation Activity in vitro of a Water-soluble Polysaccharide from Dendrobium huoshanense. J. Food Biochem. 2013, 37, 313–321. DOI:10.1111/j.1745-4514.2011.00633.x [Google Scholar]
-
Ratnayake WMN, Galli C. Fat and Fatty Acid Terminology, Methods of Analysis and Fat Digestion and Metabolism: A Background Review Paper. Ann. Nutr. Metab. 2009, 55, 8–43. DOI:10.1159/000228994 [Google Scholar]
-
Chen Y, Yang L, Wang K, An Y, Wang Y, Zheng Y, et al. Relationship between fatty acid intake and aging: A Mendelian randomization study. Aging 2024, 16, 5711–5739. DOI:10.18632/aging.205674 [Google Scholar]
-
Zhou L, Xiong JY, Chai YQ, Huang L, Tang ZY, Zhang XF, et al. Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front. Psychiatry 2022, 13, 933704. DOI:10.3389/fpsyt.2022.933704 [Google Scholar]
-
Edwards IJ, O’Flaherty JT. Omega-3 Fatty Acids and PPARgamma in Cancer. PPAR Res. 2008, 2008, 358052. DOI:10.1155/2008/358052 [Google Scholar]
-
Gani OABSM. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc. Diabetol. 2008, 7, 6. DOI:10.1186/1475-2840-7-6 [Google Scholar]
-
Li J, Lin YCD, Zuo HL, Huang HY, Zhang T, Bai JW, et al. Dietary Omega-3 PUFAs in Metabolic Disease Research: A Decade of Omics-Enabled Insights (2014–2024). Nutrients 2025, 17, 1836. DOI:10.3390/nu17111836 [Google Scholar]
-
Zou B, Zhao D, Zhou S, Kang JX, Wang B. Insight into the effects of Omega-3 fatty acids on gut microbiota: Impact of a balanced tissue Omega-6/Omega-3 ratio. Front. Nutr. 2025, 12, 1575323. DOI:10.3389/fnut.2025.1575323 [Google Scholar]
-
Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. DOI:10.3390/ijms18122645 [Google Scholar]
-
Kumar V, Rohilla A, Ahire JJ. Omega-3 fatty acids and the gut microbiome: A new frontier in cardiovascular disease prevention. Discov. Med. 2025, 2, 53. DOI:10.1007/s44337-025-00212-0 [Google Scholar]
-
Bhandari D, Rafiq S, Gat Y, Gat P, Waghmare R, Kumar V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2019, 26, 139–150. DOI:10.1007/s10989-019-09823-5 [Google Scholar]
-
Zaky AA, Simal-Gandara J, Eun JB, Shim JH, Abd El-Aty AM. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. DOI:10.3389/fnut.2021.815640 [Google Scholar]
-
Nong NTP, Hsu JL. Bioactive Peptides: An Understanding from Current Screening Methodology. Processes 2022, 10, 1114. DOI:10.3390/pr10061114 [Google Scholar]
-
Yao W, Zhang Y, Zhang G. Marine peptides as potential anti-aging agents: Preparation, characterization, mechanisms of action, and future perspectives. Food Chem. 2024, 460, 140413. DOI:10.1016/j.foodchem.2024.140413 [Google Scholar]
-
Ndinguri M, Bhowmick M, Tokmina-Roszyk D, Robichaud T, Fields G. Peptide-Based Selective Inhibitors of Matrix Metalloproteinase-Mediated Activities. Molecules 2012, 17, 14230–14248. DOI:10.3390/molecules171214230 [Google Scholar]
-
Wang J, Wu Y, Chen Z, Chen Y, Lin Q, Liang Y. Exogenous Bioactive Peptides Have a Potential Therapeutic Role in Delaying Aging in Rodent Models. Int. J. Mol. Sci. 2022, 23, 1421. DOI:10.3390/ijms23031421 [Google Scholar]
-
Apone F, Barbulova A, Colucci MG. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. DOI:10.3389/fpls.2019.00756 [Google Scholar]
-
Hao ZW, Zhang ZY, Wang ZP, Wang Y, Chen JY, Chen TH, et al. Bioactive peptides and proteins for tissue repair: microenvironment modulation, rational delivery, and clinical potential. Mil. Med. Res. 2024, 11, 75. DOI:10.1186/s40779-024-00576-x [Google Scholar]
-
Hipkiss AR, Brownson C, Carrier MJ. Carnosine, the anti-ageing, anti-oxidant dipeptide, may react with protein carbonyl groups. Mech. Ageing Dev. 2001, 122, 1431–1445. DOI:10.1016/S0047-6374(01)00272-X [Google Scholar]
-
Qiu Z, Yan M, Li Q, Liu D, Van Den Steen PE, Wang M, et al. Definition of peptide inhibitors from a synthetic peptide library by targeting gelatinase B/matrix metalloproteinase-9 (MMP-9) and TNF-α converting enzyme (TACE/ADAM-17). J. Enzym. Inhib. Med. Chem. 2011, 27, 533–540. DOI:10.3109/14756366.2011.599323 [Google Scholar]
-
Hipkiss AR, Brownson C. A possible new role for the anti-ageing peptide carnosine. Cell. Mol. Life Sci. (CMLS) 2000, 57, 747–753. DOI:10.1007/s000180050039 [Google Scholar]
-
Jiang SJ, Tsai PI, Peng SY, Chang CC, Chung Y, Tsao HH, et al. A potential peptide derived from cytokine receptors can bind proinflammatory cytokines as a therapeutic strategy for anti-inflammation. Sci. Rep. 2019, 9, 2317. DOI:10.1038/s41598-018-36492-z [Google Scholar]
-
Magrone T, Jirillo E. Nutraceuticals in immunosenescence. In Anti-Ageing Nutrients, 1st ed.; Neves D, Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 183–202. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118823408.ch5 (accessed on 15 November 2025).
-
Ding X, Ma X, Meng P, Yue J, Li L, Xu L. Potential Effects of Traditional Chinese Medicine in Anti-Aging and Aging-Related Diseases: Current Evidence and Perspectives. Clin. Interv. Aging 2024, 19, 681–693. DOI:10.2147/CIA.S447514 [Google Scholar]
-
Dhanjal DS, Bhardwaj S, Sharma R, Bhardwaj K, Kumar D, Chopra C, et al. Plant Fortification of the Diet for Anti-Ageing Effects: A Review. Nutrients 2020, 12, 3008. DOI:10.3390/nu12103008 [Google Scholar]
-
Reis Da Silva TMH. Herbal Solutions for Longevity: Anti-Ageing Compounds From Traditional Medicine. In Drug Discovery and Antiaging Approaches for Human Longevity; Chen JT, Ed.; IGI Global Scientific Publishing: Palmdale, PA, USA, 2025; pp. 325–350. Available online: https://services.igi-global.com/resolvedoi/resolve.aspx?doi=10.4018/979-8-3693-9730-5.ch009 (accessed on 15 November 2025).
-
Naik S, Lagishetty C. Polyamines: Potential anti-inflammatory agents and their possible mechanism of action. Indian J. Pharmacol. 2008, 40, 121. DOI:10.4103/0253-7613.42305 [Google Scholar]
-
Li W, Chen H, Xu B, Wang Y, Zhang C, Cao Y, et al. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Future Foods 2023, 3, 289–305. DOI:10.1016/j.jfutfo.2023.03.001 [Google Scholar]

