The Heterogeneity and Functional Roles of Dendritic Cells in Atherosclerosis: Origins, Subsets, and Therapeutic Implications
Received: 19 July 2025 Revised: 26 August 2025 Accepted: 29 September 2025 Published: 10 October 2025
© 2025 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Atherosclerosis, a chronic inflammatory arterial disease, represents the primary pathological basis for cardiovascular diseases (CVDs)—the leading global cause of mortality and morbidity [1,2]. While immune dysregulation has been well-established as a critical driver of atherosclerotic lesion development, progression, and plaque instability[3,4], the specific contributions of DCs within the complex immune microenvironment (comprising macrophages, T/B lymphocytes, NK/NKT cells, neutrophils, and mast cells) remain poorly characterized [5,6]. This knowledge gap persists despite compelling evidence of DC presence in both healthy arterial intima and atherosclerotic lesions across human carotid arteries and murine models [7,8,9,10,11], along with their demonstrated capacity to orchestrate dual atheroprotective and atherogenic immune responses [12,13,14,15,16,17].
Emerging clinical and preclinical data reveal that DC accumulation correlates with atherosclerotic progression in humans, rodents, and mice [18,19,20,21], with mechanistic studies implicating DCs in regulating tertiary lymphoid organ formation and lesion dynamics [22]. Although recent advances have identified distinct DC subsets with specialized roles in atherogenesis [23,24], the precise molecular mechanisms governing their context-dependent pro- and anti-inflammatory functions remain incompletely elucidated.
This review systematically examines: Functional heterogeneity of DC subsets in atherosclerosis pathogenesis, including subset-specific biomarkers; Crosstalk between DCs and key lesion cellular components (T cells, B cells, ECs, smooth muscle cells); Genetically engineered murine models for DC research; Emerging DC-targeted immunotherapeutic strategies. This comprehensive analysis aims to clarify DC biology in atherosclerotic progression and highlight translational opportunities in DC-based therapeutics.
2. Origin and Development of DCs in Atherosclerosis
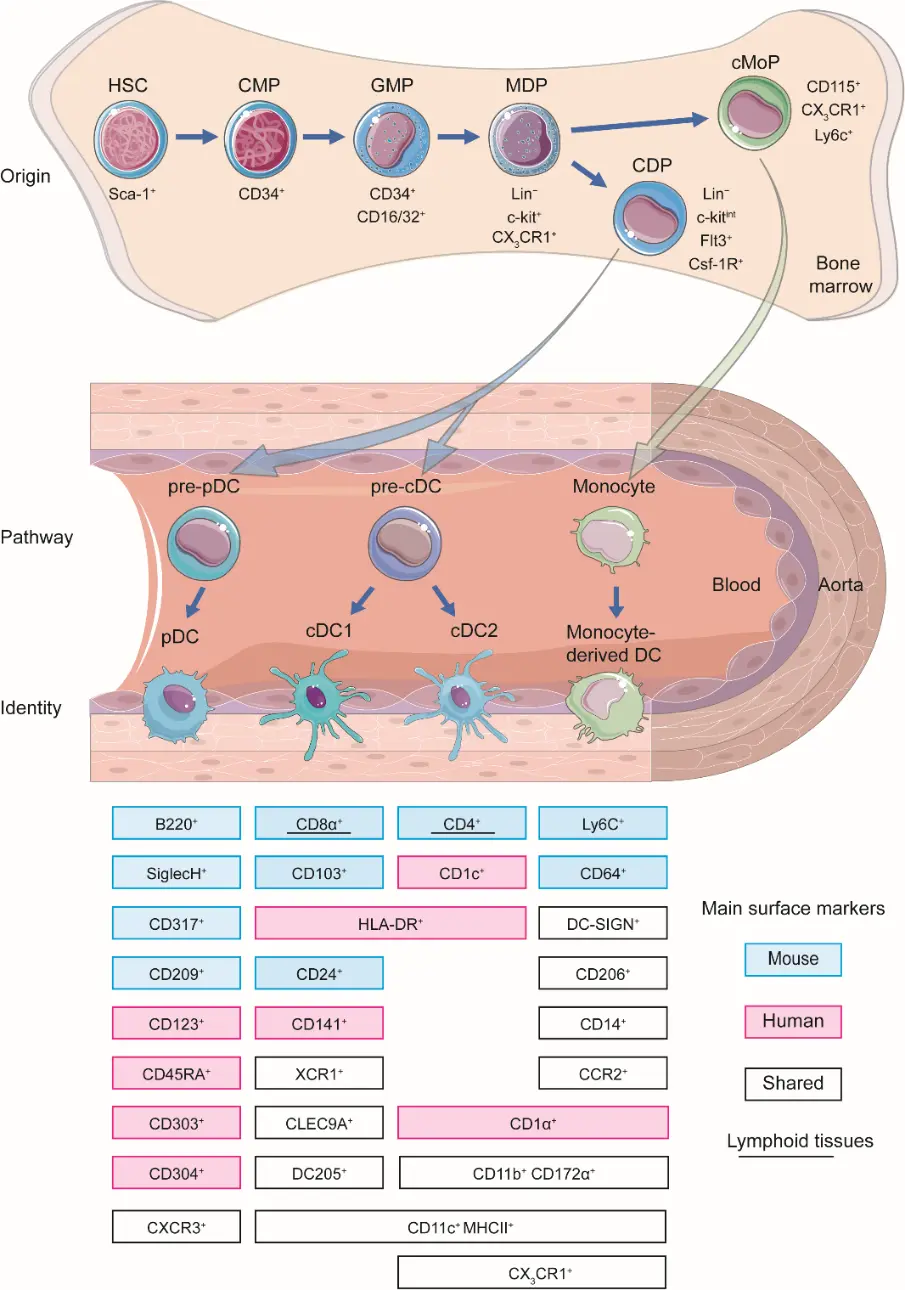
Since DCs discovery by Steinman et al. in 1973, their ontogeny has been extensively characterized [25]. DCs originate from hematopoietic stem cells (HSCs) in the bone marrow, progressing through a hierarchical differentiation pathway via common myeloid progenitor (CMP, CD34+), granulocyte/macrophage progenitor (GMP, CD34+CD16/32+), macrophage-dendritic progenitor (MDP, Lin−c-kit+CX3CR1+), common DC precursors (CDP, Lin−c-kitintFlt3+Csf-1R+) [26]. The CDP bifurcates into two primary lineages, plasmacytoid DCs (pDCs) and conventional DCs (cDCs). The cDC lineage further diversifies into two major subsets: cDC1 and cDC2, each with distinct developmental dependencies (e.g., FLT3L, IRF8, BATF3 for cDC1; IRF4, ZEB2 for cDC2) and functional specializations [27]. Beyond this canonical pathway, DCs can also derive from monocytes under inflammatory conditions, highlighting their ontogenetic plasticity [28] (Figure 1).
In the context of Atherosclerosis, DCs are believed to originate from both the local activation of resident cells within the vessel wall [29,30] and the recruitment of circulating precursors (e.g., pre-DCs, monocytes) [31]. Damage-associated molecular patterns (DAMPs) released from injured ECs [32,33], necrotic smooth muscle cells [34], apoptotic macrophages [35], destabilized extracellular matrix components (e.g., fragmented collagen, oxidized LDL) [36], local cytokines and chemokines (e.g., CCL2, CCL20, GM-CSF, FLT3L, IFN-γ), orchestrate the homing and subsequent maturation of these cells within the atherosclerotic niche [37]. Mature DCs migrate to secondary lymphoid organs via CCR7 (C-C Motif Chemokine Receptor 7) to prime naive T cells, bridging innate and adaptive immunity [37]. The specific contributions of the different DC subsets, cDC1, cDC2, pDCs, and moDCs, to atherogenesis are complex and context-dependent. The subsequent sections discuss their detailed roles in modulating T cell responses (e.g., Th1, Th2, Th17, Treg) and overall plaque fate.
3. Characterizing DC Subsets
In mice, DCs represent a functionally diverse population within the mononuclear phagocyte system. Vascular DCs uniformly express CD11c [12,24,38,39] and are identified through a combination of lineage-specific markers. As highly professional antigen-presenting cells (APCs), vascular DCs exhibit versatile antigen-presenting capabilities that support adaptive immune responses. They are proficient at priming naive CD4+ T-cell activation and proliferation via strong MHC II–dependent interactions [40], and priming naive CD8+ cytotoxic T-cell responses by loading exogenous antigens onto MHC I molecules [41]. Multiple phenotypically distinct vascular DC subsets have been mapped to the arterial intima in both healthy individuals and murine atherogenic hotspots, highlighting their spatial and functional diversity [7,8,12,17,21]. As summarized in Table 1, vascular DCs are broadly classified into three subsets based on ontogenetic origin and function: cDCs, pDCs, and Mo-DCs [12,24].
Table 1. Phenotype and ontogeny of mouse and human vascular DCs.
Categories |
Main Surface Markers |
Main PRRs |
Antigen Presentation |
Cytokines |
||
|---|---|---|---|---|---|---|
Mouse DC |
Mo-DC |
CD11c++ MHCII+++, CD11b, CD209, CD206, CD64, CD14, CD172a, DC-SIGN, CX3CR1, F4/80 |
Not well defined |
Cross presentation to CD8+ T cells, direct presentation to CD4+ T cells |
IL-12 IL-23 TNF iNOS |
|
cDC |
cDC1 |
CD11c+++ MHCII+++, CD8a, CD103, CD24, CD205, XCR1, CLEC9A |
TLR2-4, TLR11-13, RLR, STING, Clec12a |
Cross presentation to CD8+ T cells, direct presentation to CD4+ T cells |
IL-12 IFN-III |
|
cDC2 |
CD11c+++ MHCII+++, CD4, CD11b, CD172a, |
TLR1-2, TLR4-9, TLR13, RLR, STING, CLEC4A, CLEC6A CLEC7A |
Direct presentation to CD4+ T cells |
IL-6 IL-23 TNF |
||
pDC |
CD11c++ MHCII+, SiglecH, CD317, CCR9, B220, Ly49Q |
TLR7, TLR9, TLR12, RLR, STING, CLEC12A |
Limited antigen presentation ability |
IFN-I IFN-III |
||
Human DCs |
Mo-DC |
CD11c++ MHCII+++, CD11b, CD209, CD206, CD1a/b/c, CD14 |
Not well defined |
Direct presentation to CD4+ T cells, inducing Th1 and Th17 responses |
IL-1β IL-6 IL-12 IL-23 IL-10, TNF iNOS |
|
cDC |
cDC1 |
CD11c+++ MHCII+++, CD141, XCR1, CLEC9A |
TLR1, TLR3, TLR6, TLR8, TLR10, STING, CLEC12A |
Cross presentation to CD8+ T cells, direct presentation to CD4+ T cells, induction of Th1 responses |
IL-12 TNF IL-6 IFN-I IFN-III |
|
cDC2 |
CD11c+++ MHCII+++, CD1c, CD172a, CD11b |
TLR1-9, RLR, NLR, STING, CLEC4A, CLEC6A, CLEC7A, CLEC10A, CLEC12A, |
Direct presentation to CD4+ T cells, inducing Th2 and Th17 responses and Tregs |
IL-1β IL-6 IL-12 IL-23 IL-10 TNF TGF-β |
||
pDCs |
CD11c– MHCII+++, CD123, CD303, CD304, CD45RA, |
TLR7, TLR9, RLR, STING, CLEC12A |
Poor in antigen presentation |
IFN-I IFN-III TNF |
||
Notes: 1. DC-SIGN: DC-specific ICAM3-grabbing non-integrin; 2. TLR: Toll-like receptor; 3. IL: Interleukin; 4. TNF: Tumor necrosis factor; 5. IFN: Interferon; 6. CLEC9A/12A: C-type lectin domain containing 9A/12A; 7. RLR: RIG-I like receptors; 8. STING: Stimulator of interferon genes; 9: TGF-β: Transforming growth factor beta.
3.1. cDCs
cDCs derived from CDPs of the HSCs, which migrate into tissues via blood as pre-DCs, where they then locally differentiate into DCs [42]. cDCs are dedicated APCs and characteristiced by dendritic morphology and high expression of MHC class II molecules, and can proliferate in early lesions [29]. Based on phenotypic characteristics and ontogeny, cDCs can be broadly classified into two distinct main categories, cDC1 and cDC2, wherein subsets within each category share consistent similarities in development and function. Notably, a largely analogous classification system for cDCs exists in mice and humans[43,44]. In general, cDCs accumulate in mouse and human atherosclerotic lesions with a marked increase in advanced stages and in complicated plaques [45].
cDC1 subset expresses the transcription factor Batf3 and is specialized in presenting cell-associated antigens derived from intracellular pathogens. In preclinical models, a particular DC subset, cDC1 is shown to be specialized in cross-presenting extracellular antigens to CD8+ T cells [46] and activating a type I immune response. The most commonly used marker to identify cDC1 is CD8α in mice and CD141 (also known as BDAC3) in humans. Other markers that can be used in combination with CD141 to identify cDC1 include CD103 [47], XCR1 [48], CLEC9A [49], and CADM1 [50].
cDC2 express the transcription factor IRF4 and are specialized in presenting antigens from extracellular pathogens [51], such as bacteria and parasites. They are present in atherosclerotic plaques and can promote the activation of CD4+ T cells [52], which are important for coordinating the immune response against these pathogens. The characteristic markers for cDC2 are CD11b and CD172a (also known as SIRPα) in mice [53], and CD1c (also known as BDAC-1) in humans.
Based on tissue localization and migratory pathways, the cDCs can be divided into migratory DCs and lymphoid tissue-resident DCs [54,55]. Migratory DCs are a subset of DCs that have the ability to migrate from peripheral tissues to lymph nodes, where they interact with T cells to initiate an immune response [56]. These DCs are characterized by their expression of specific chemokine receptors, such as CCR7, which allows them to respond to chemotactic signals produced by lymphatic ECs [57]. Migratory DCs are important for the induction of adaptive immune responses against pathogens and tumors, and they play a critical role in immune surveillance and tolerance [58,59]. They are also involved in the pathogenesis of autoimmune diseases and allergy [60]. Lymphoid tissue resident DCs are a subset of DCs found in lymphoid tissues, such as lymph nodes, spleen, and Peyer’s patches [61]. They play a critical role in maintaining immune homeostasis by regulating the activation and differentiation of T cells [62]. Lymphoid tissue-resident DCs are characterized by their expression of specific surface markers, such as CD8α in mice and CD141 in humans for cDC1s [63], and CD11b in mice and CD1c in humans for cDC2s [64]. They are also involved in initiating and regulating immune response against pathogens and tumors, and play a critical role in developing immune tolerance [65,66,67].
3.2. pDCs
pDCs are unique bone-marrow-derived cells, and have been found to reside primarily in the adventitia of arteries [13], and also identified in the shoulder regions of plaques in mice [45] and humans [8] in small numbers. Unlike cDCs, pDCs are a small subset of DCs and are poor in antigen presentation, and specialized in producing large amounts of type I interferons [68], which is dependent on the interferon response family transcription factors, including IRF7 [69]and IRF8 [70]. pDCs derive directly from CDPs in the bone marrow in a Flt3L-dependent manner, and the subsequent specification requires the E protein transcription factor TCF4 (also known as E2-2) [71,72]. pDCs are identified by expression of B220 (also known as CD45R), PDCA-1 (also known as blood pDC antigen 1 or CD317) and Siglec-H (sialic acid-bing immunoglobulin-like lectin H) in mouse lesions [73,74], and CD123 (also known as IL-3 receptor alpha chain), BDCA-2 and BDCA-4 (also known as blood DC antigen 2 and 4) in human plaques [75,76].
3.3. Mo-DCs
In the steady state, Mo-DCs are scarcely identified in mucosal tissues in both mouse and human [77,78,79]. However, monocytes can give rise to cells with many of the phenotypic and functional features of DCs in an inflammatory status. Circulating monocytes from peripheral blood can rapidly mobilize and differentiate into Mo-DCs and acquire potent antigen-presenting capacity via upregulating CD11c and MHCII molecules [80]. Contrary to cDCs, Mo-DCs have a higher CX3CR1 and DC-specific ICAM3-grabbing non-integrin (DC-SIGN) expression [12], and they can be distinguished from resident CD11b+ DCs via Ly6c and Mac3 [81]. Mo-DCs express markers such as CD80, CD86, and HLA-DR [82], which are typical of mature DCS, and also express several DC-restricted markers [83], such as MIDC-8, CD172a, and CD209a [84,85,86]. In addition, a recent study identified other makers expressed in Mo-DCs, such as NAPSA, IFI30, and IFITM1, which play an essential role in the formation of functional blood vessels [87]. Like classical monocytes, Mo-DCs arise independently of FLT3 and instead are dependent on the cytokine macrophage clony-stimulating factor (M-CSF) [88]. They do not express the transcription factor ZBTB46 and depend on the chemokine receptor CC-chemokine receptor 2 (CCR2) for recruitment into inflammatory sites, a mechanism shared with classical monocytes and pre-cDCs [89,90].
4. Functional Role of Vascular DCs in Atherosclerosis
DCs, whether in lymphoid or nonlymphoid tissues, comprise only a minor portion of total cell population, and also scarce in human and mouse plaques, but they provide a unique role in the development and progression of atherosclerosis.
4.1. cDCs
cDCs are specialized in antigen presentation, with cDC1 excelling at cross-presenting exogenous antigens on MHC-I to activate naive CD8⁺ T cells, and cDC2 primarily presenting antigens on MHC-II to activate naive CD4⁺ T cells. Both subsets respond to antigens derived from oxLDL and are potent producers of pro-inflammatory cytokines, such as TNF-α [91], IL-6 [92], and IL-12 family [93], that promote Th1 and Th17 differentiation and activation [94]. Recent lineage-tracing studies underscore their subset-specific roles: cDC1s drive Th1 responses [95], while cDC2s promote Th2 [96], Th17 [97], and Treg responses [64], implicating DC heterogeneity as a critical determinant of plaque fate [27,28]. The ultimate role of cDCs in atherosclerosis is context-dependent. cDC1s typically promote pro-atherogenic immune responses through cross-presentation to CD8⁺ T cells and interactions with NK cells and B cells [98]. However, they also exhibit functional plasticity and may contribute to tolerogenic responses in early disease stages or specific microenvironments. This duality is influenced by disease stage, tissue location, and local inflammatory signals. Meanwhile, cDC2 cells can promote the differentiation of naive T cells into various helper subsets—including Th1, Th2, Th17, Tfh, and Treg cells [99]. These subsets perform distinct functions: Th1, Th2, and Th17 cells produce pro-inflammatory cytokines such as IFN-γ, IL-4/IL-5/IL-13, and IL-17, respectively [100,101,102], whereas Treg cells exert immunosuppressive effects [103].
4.2. pDCs
Clinical studies have confirmed that pDCs exist in the shoulder region of human plaques, and the reduced number of pDCs is one predictor of cardiovascular events[104]. Due to low expression of MCH II and co-stimulatory molecules, pDCs are known as poor T cell activators. But the capacity of pDCs to phagocytose and prime antigen-specific T cell responses could be enhanced in mouse and human atherosclerotic lesions, when exposed to ox-LDL [105], and depletion of pDCs promotes plaque T cell accumulation and exacerbates lesion development and progression [68]. Further study suggested that pDCs may contribute to the progression of atherosclerosis by recruiting macrophages to the arterial wall [106] and stimulating cytotoxic T cell function in the lesions via producing type I interferons [107]. However, other studies have suggested that pDCs may also have a protective role in atherosclerosis by promoting Treg differentiation and dampening T cell proliferation and activity via the release of tolerogenic enzyme indoleamine 2,3-dioxygenase (IDO) in peripheral lymphoid tissue [68]. Another study indicated that pDCs may affect the development of atherosclerosis by inducing the maturation of cDCs and the recruitment of macrophages [106,108]. Overall, the role of pDCs in atherosclerosis is context-dependent. Within the inflammatory plaque, pDCs are activated by DAMPs (e.g., ox-LDL) via TLRs to produce type I IFNs, which exacerbate disease by recruiting macrophages and activating T-cells. Conversely, in lymphoid tissues, pDCs can exert a protective effect by producing IDO to suppress T-cell proliferation and promote Treg differentiation. This functional duality explains apparent contradictions in the literature, which may also be influenced by factors such as disease stage and methodological approaches used to study pDCs.
4.3. Mo-DCs
Several studies proved that Mo-DCs are a crucial reservoir of APCs [109], and the major producer of pro-inflammatory cytokines, and can induce Th1 differentiation [110] and activate memory T cell [111]. Based on the intrinsic properties, Mo-DCs can differentiate into two distinct subsets: inflammatory DCs, which promote inflammation and immune cell activation by presenting antigens to T cells and secreting pro-inflammatory cytokines [112], and tolerogenic DCs, which induce immune tolerance by presenting antigens to regulatory T cells and secreting anti-inflammatory cytokines [113,114].
The accumulation and maturation of Mo-DCs in the arterial wall could be enhanced via interaction with platelets [115]. Mo-DCs can emigrate from the arterial wall and atherosclerotic lesions at the early stage of atherosclerosis [116]. Still, little emigration has been detected from progressive lesions, indicating that plaque progression may result not only from the robust monocyte recruitment into arterial walls but also from the reduced emigration of DCs from lesions [117]. Conversely, in some cases, egress of Mo-DCs from lesions with a fragile morphology may also contribute to plaque rupture or disruption rather than lead to an atheroprotective benefit [45,118]. Defective egress of DCs from the aorta and their altered trafficking toward lymph nodes can lead to excessive accumulation of Mo-DCs in the atherosclerotic lesions [14].
4.4. DCs Engulf Lipids and Control Cholesterol Homeostasis
Although macrophages are the main participants in lipid take and foam cell formation, resident intimal DCs could also rapidly engulf lipid and become the first foam cells in nascent lesions [119]. However, the percentage of DC-derived foam cells in atherosclerotic lesions is unclear. Several studies point toward a role of DCs in cholesterol metabolism. CD11c+ DCs enhance lipid uptake and clearance in circulation to lower LDL levels, but CD11c is also expressed by certain macrophages and other immune cells [120]. Thus, depletion of CD11c+ cells (which may include non-DCs) increases the plasma cholesterol level [98]. However, the depletion of Flt3-dependent DCs in Flt3−/−Ldlr−/− mice did not change lipid levels [12]. Further lineage-tracking systems may be an excellent method to solve this question.
DC-derived foam cell could precede endothelial cell activation and increased monocyte recruitment and enhance the presentation of lipid and peptide antigens to NKT and T cells [14]. Ox-LDL uptake by DCs induces upregulation of scavenger-receptors, maturation and differentiation via LOX1-mediated MAPK/NF-kappaB pathway [121], which promotes the initiation and progression of immune cell activation in atherosclerotic lesions [122]. Another study suggested enhanced cytokine production was observed in ox-LDL stimulated DCs, which are activated through binding of CD36 and TLR4 [123]. Future studies aiming at enhancing the lipid-lowering potential of DC could be a potential strategy for the treatment of atherosclerosis.
4.5. DCs Initiate of Native T Cell and T Cell Differentiation and Development
Several lines of evidence illustrate that the interaction of DCs and T cells causally contributes to atherogenesis. For example, a deficiency of the invariant chain of MHCII could reduce atherosclerosis in Ldlr−/− mice [124], and abrogating transforming growth factor beta receptor II signaling in DCs promotes immune reactivity of T cells resulting in enhanced atherosclerosis [125]. Recent studies have further linked DCs with Treg responses in atherosclerosis. CCL17-expressing cDC1 drives atherosclerosis via constraining Treg-maintenance [126], deficiency of aortic cDC1 diminishes Treg in the aorta of Ldlr−/− mice [12], and dysfunctional DCs lead to a loss in Tregs. Further clinical studies confirmed that peripheral Treg numbers were reduced and CCL17 serum levels were increased in CAD patients, which may be in line with the expansion of CCL17+ DCs in atherosclerosis [127,128]. While DCs can emigrate from atherosclerotic lesions after antigen uptake and home to lymphatic tissue in a CCR7-dependent manner, they may also recruit T cells to the inflamed vessel wall via secretion of CCL17, CCL19, or CCL20 [129]. They can activate T cells and induce their proliferation and differentiation into effector T cells, leading to the promotion of inflammation and immune cell activation in the arterial wall [125]. The differentiation of T cells into different subsets, such as Th1, Th2, Th17, and Treg cells, can also be influenced by the cytokine milieu and other signals provided by the DCs [94].
4.6. DCs Induce Immune Tolerance
DCs play a critical role in the maintenance of immune tolerance, which is the process by which the immune system is trained to recognize and tolerate self-antigens while mounting effective responses against foreign antigens [130]. The role of DCs in the induction of immune tolerance in atherosclerosis is an area of ongoing research. Recent studies have proved that DCs can induce the differentiation of Tregs, which play a protective role in atherosclerosis by suppressing immune response and preventing autoimmunity [131]. Vaccination of LDL receptor deficient mice with DCs transfected with foxp3 encoding mRNA reduced Tregs and aggravated atherosclerosis [132]. Some studies found that injection of DCs loaded with oxLDL or ApoB100 into atherosclerotic mice reduced atherosclerotic lesions and increased Tregs [133,134].
4.7. DCs Regulate B Cell Development, Differentiation and Activation
B cells were rarely found in atherosclerotic plaques, but vascular DCs were found to form direct contact with them [135]. DCs can present antigens to B cells, activating and differentiating them into antibody-secreting plasma cells. DCs can also secrete cytokines, such as IL-6 and IL-10, which can influence B cell differentiation and activation [136]. On the other hand, B cells can modulate the function of DCs by producing cytokines, such as BAFF and APRIL, which can promote the survival and maturation of DCs [137]. B cells can also present antigens to DCs, leading to their activation and the promotion of immune responses. The interaction between DCs and B cells in atherosclerotic lesions can contribute to the development of immune responses against modified lipoproteins and other antigens present in the arterial wall, promoting inflammation and the progression of atherosclerosis [138].
More recently, a proatherogenic role has been attributed to the newly identified innate response activator B cells by promoting the expansion of cDCs and polarization [139].
4.8. The Relationship between DCs and ECs in Atherosclerotic Lesions
ECs line the inner surface of blood vessels and act as a barrier between the bloodstream and underlying tissues, regulating the passage of substances and immune cells [140]. This endothelial dysfunction is characterized by the increased expression of adhesion molecules, the secretion of pro-inflammatory cytokines, and altered permeability, which together create a conducive environment for leukocyte recruitment and retention. The basic aspect of DC function is its capacity to adhere and migrate through ECs [141]. The interplay between these two cell types is crucial for the pathophysiology of atherosclerotic lesions, influencing both inflammation and immune responses in the arterial environment. During the development of atherosclerosis, ECs become activated and produce adhesion molecules and chemokines that promote the recruitment of immune cells, including DCs, to the site of the developing plaque [142,143].
DCs need to tether and adhere to ECs to exit from peripheral blood for antigen acquisition, then infiltrate into the subendothelial space [144,145]. Various mechanisms mediate the interaction of DCs and ECs, including adhesion molecules and chemokine receptors [146,147]. Chemotactic stimuli and the involvement of adhesion molecules are necessary to invade DCs into the intima [141]. For example, mature DCs express the chemokine receptor CCR7, which interacts with its ligands CCL19 and CCL21 produced by activated ECs, allowing DCs to migrate to the lymph nodes where they can initiate an immune response [148,149]. And the multiple factors which accelerate atherogenesis could also enhance the DCs binding to and transmigration through the endothelial cell layer, such like TNF-α, ox-LDL, hypoxia and inhibition of NO synthesis [141]. In addition, DCs can promote endothelial cell dysfunction and inflammation by producing pro-inflammatory cytokines and chemokines, such as TNF-α and IL-6 [150], which can activate ECs and promote the expression of adhesion molecules and chemokines [145].
4.9. The Relationship between DCs and Vascular Smooth Muscle Cells (VSMCs) in Atherosclerotic Lesions
VSMCs are the predominant cell type in the arterial wall and responsible for maintaining the structural integrity and contractile function of blood vessels[151]. During atherosclerosis development, the interaction between DCs and SMCs may contribute to the progression of plaque inflammation, remodeling, and stability[152]. DCs can promote the activation of SMCs by producing pro-inflammatory cytokines and chemokines, such as IL-6 and MCP-1, which can induce SMCs migration and proliferation [153]. Moreover, DCs can also induce SMC apoptosis, which can contribute to the destabilization of the plaque and the rupture of the fibrous cap [154], and partially explained the phenomenon that up to 70% of DCs were accumulated in the shoulders of vulnerable plaques [45], On the other hand, SMCs can modulate the function of DCs by producing cytokines and growth factors that can influence DC maturation and activation. For example, SMCs can produce TGF-β, which can promote the differentiation of tolerogenic DCs that induce immune tolerance.
In addition, one study has observed Lag-antibody positive cells in the aortic wall [155], and another study demonstrated that epidermal Langerhans cells could prevent UVB (ultraviolet radiation B) exposure-inhibited atherosclerosis development via suppression of proatherogenic T cell responses [156]. These data suggested that the close relationship between vascular DCs and Langerhans cells may also be involved in atherosclerosis.
5. Clinical Application
As DCs play a crucial role in the immune system by initiating and directing immune response, previous studies manifested that DCs have the potential to be used as a therapeutic toll in various diseases, including cancer [157,158,159], autoimmune disorders [160,161,162], and infectious diseases [163,164,165,166,167]. While the clinical application of DCs in atherosclerosis is still under investigation, several approaches have been explored.
5.1. DC-Based Vaccine
Induced tolerogenic DCs are a powerful immunotherapy for autoimmune disease, which have shown promise in clinical trials [168,169,170]. One of the approaches used to design DC-based vaccines for atherosclerosis is to load DCs isolated from patients and loaded with self-antigens, such as ox-LDL [171,172,173,174], heat shock proteins [175,176,177], apoB100 [133,178,179,180]and other plaque-associated antigens [181,182], can lead to a reduction of lesion size via dampening T cell activation and pro-inflammatory cytokine production, or inducing the differentiation of regulatory T cells, which can suppress immune responses against the arterial wall and reduce inflammation [133,183]. The activated DCs can then be delivered back to mouse or patient to decrease Th1 response and increase specific antibodies against these antigens, potentially reducing plaque formation and inflammation [134,184].
Another approach is to modify DCs to induce immune tolerance towards atherosclerosis. This can be achieved by using cytokines or factors to induce a regulatory DC phenotype that produces anti-inflammatory cytokines such as IL-10 [185]and TGF-β [186] via inducing regulatory T cells. This type of DC has been shown to reduce atherosclerotic lesion size and stabilize plaques in animal models.
Moreover, a DC-targeting nasal double DNA adjuvant system could be an effective nasal immunization strategy for preventing atherosclerotic lesion accumulation in the aortic sinus, mediated by inducing atheroprotective IgM antibodies via DC-B-1a B cell interactions [187]. In addition, DC-based vaccines have been combined with other approaches, such as statin therapy or adoptive transfer of regulatory T cells, to enhance their therapeutic effect further.
5.2. Therapeutic Modulation of Dendritic Cell Function and Signaling
Therapeutic strategies aimed at modulating dendritic cell (DC) function represent a promising approach to dampen inflammation and curb the progression of atherosclerosis. This can be achieved through several mechanisms, including the use of pharmacological agents to influence DC activation state or by targeting specific signaling pathways and receptors critical for their pro-inflammatory functions.
A broad range of conventional drugs has been shown to attenuate atherosclerosis, in part by indirectly modulating dendritic cell (DC) activation and maturation. It is important to note that the effects of these agents, which include conventional lipid-lowering drugs [188,189,190,191], HMG-CoA reductase inhibitors [188], PPAR agonists [192,193], and traditional Chinese medicines [194,195,196,197], and vitamin D receptor agonists [198], are pleiotropic. They exert their benefits by systemically altering the metabolic and inflammatory milieu (e.g., reducing hypercholesterolemia and oxidative stress), which in turn influences the function of multiple immune cell types, including monocytes, macrophages, T cells, and DCs. Consequently, while observed changes in DC phenotype following such treatments (e.g., suppressed maturation or a shift towards tolerance) are functionally important, they may often represent an indirect effect secondary to global changes in the immune environment rather than direct DC-specific targeting.
In contrast to these broad-acting therapies, a more precise strategy involves the direct targeting of specific molecules or receptors on DCs that are central to the inflammatory response. DCs express a repertoire of pattern-recognition and signaling receptors, including TLRs [199,200,201], TREM-1 [202,203], DC-SIGN [204,205,206], and TGFβRII [125], that recognize danger signals and orchestrate immune activation. Consequently, selectively modulating signaling through these receptors, such as by inhibiting specific TLR or TREM-1 pathways, may help regulate the immune response and reduce plaque inflammation in a more targeted manner [207]. Furthermore, selective modulation of autophagy in DCs has been identified as an intriguing therapeutic target [154,174]. Future efforts to develop truly DC-specific therapies, such as nanocarriers for subset-specific delivery or antibodies against unique surface receptors, offer a promising path toward precise immunomodulation with reduced systemic effects. Beyond small molecules, biologic agents such as exogenous IL-37 can inhibit DC maturation, induce regulatory immune responses, and attenuate disease in Apoe−/− mice via the IL-1R8-TLR4-NF-κB pathway [208].
Looking ahead, DC-based vaccines show promise for atherosclerosis treatment [209], and inhibiting the recruitment of pathogenic DC subsets may help limit lesion growth and local inflammation. As atherosclerosis is multifactorial, further research is needed to characterize DC subsets, optimize intervention design and delivery of vaccines, evaluate safety, and ultimately assess efficacy in clinical trials. Combination therapies incorporating DC-targeted strategies with other treatments may yield optimal outcomes. Ultimately, targeted modulation of DC function remains an active research area with significant potential for advancing therapeutic options against atherosclerosis.
5.3. Emerging Insights from Human Studies and Translational Challenges
Advancements in single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics refine our understanding of human atherosclerotic plaques, providing unprecedented resolution of cellular heterogeneity and revealing rupture-prone microenvironments [210,211,212]. These technologies offer critical insights into human-specific DC involvement and potential therapeutic targets, helping to bridge the gap between animal models and human disease.
DC-based vaccines and targeted DC modulation hold translational potential, yet considerable challenges remain. Tolerogenic DC vaccines loaded with atherosclerosis-relevant antigens have shown promise in preclinical models by expanding regulatory T cells and reducing plaque inflammation [133,172,174,178,179,180]. Similarly, specific pharmacological agents or biologics can modulate DC function toward anti-inflammatory phenotypes [154,174,208]. A primary challenge involves the identification of human-specific therapeutic targets and biomarkers through deep molecular profiling of plaques across disease stages. Furthermore, manufacturing clinical-grade cell products or targeted therapies remains complex and costly, demanding rigorous standardization. Safety concerns, particularly the risk of disrupting broader immune function, also necessitate careful evaluation. Future progress will likely depend on integrating multi-omics data from human studies, developing standardized protocols for therapeutic development, and designing innovative clinical trials that can effectively evaluate both immunological and clinical outcomes in appropriate patient populations.
6. Animal Models Used for DC Research
A variety of animal models are used in atherosclerosis research, including non-human primates [213,214], pigs [215,216], rabbits [217], zebrafish [218,219], rats [220], and mice [221]. Among these, mice have become the predominant model organism due to their lower cost and superior genetic tractability. The two most widely used hypercholesterolemic mouse models are Apoe−/− and Ldlr−/−mice [222]. Apoe−/− mice develop spontaneous plaques on a chow diet, while Ldlr−/− mice typically require a high-fat diet to induce significant hyperlipidemia and lesion formation. Crossing gene-modified mice with these models has proven highly valuable for elucidating the roles of specific dendritic cell (DC) subsets in atherosclerosis.
Early studies predominantly utilized conventional knockout mice, including Batf3, Flt3L, IRF8, CCR2, CCR7, Clec9a and Clec4a4, CD40 and CD11c-DTR mice (Table 2). A major limitation of these models is that systemic gene deletion affects multiple cell types, making it difficult to attribute phenotypic changes exclusively to DCs. Despite this constraint, these foundational studies revealed essential and non-redundant roles for DCs by targeting specific biological processes, including development (e.g., Batf3, Flt3L, IRF8) [41,223,224,225,226,227,228,229], migration (e.g., CCR2, CCR7) [230,231,232,233,234], and immunogenic function (e.g., CD40, Clec9a) [98,134]. For instance, Batf3−/− mice underscores the complex role of cDC1s, which can be either pro-atherogenic by promoting Th1 responses or neutral, highlighting the importance of disease stage and metabolic environment. Conversely, deficiency in IRF8 or Clec9a (DNGR-1) attenuates disease, revealing the critical role of cDC1s in bridging innate cell death to adaptive Th1 immunity. The atheroprotective effect of Clec4a4 (a cDC2-specific receptor) deletion further demonstrates functional specialization within the DC lineage, suggesting subset-specific pathways that could be therapeutically targeted. The profound atherosclerosis exacerbation upon broad ablation of CD11c+ cells in CD11c-DTR model, despite the concomitant loss of some macrophages, unequivocally established the net protective role of the CD11c+ compartment, likely through immune-regulatory mechanisms. Recent advances in genetic tools have enabled more precise targeting of DC subsets. The identification of specific DC markers has facilitated the development of conditional transgenic strains using CRISPR/Cas9 technology, such as CD11c-Cre, Zbtb46-Cre, Clec9a-Cre, and Xcr1-Cre (Table 3), offering improved specificity in interrogating DC functions.
Table 2. Summary of DC deficient mouse models for AS research.
|
Model |
Core Function |
Atherosclerosis Phenotype & Mechanism |
References |
|---|---|---|---|
|
Batf3 |
Essential transcription factor for cDC1 development |
• Apoe⁻/⁻ background: ↑ Lesions (↓ splenic CD8⁺ DCs/aortic CD103⁺ cDC1, ↓ Th1) • Ldlr⁻/⁻ background: no effect on lesion size |
|
|
Flt3L |
Critical cytokine for DC development |
↓ cDC1/cDC2/pDCs (lymphoid/non-lymphoid tissues), progenitors (CDP/CLP) |
|
|
IRF8 |
Transcription factor regulating DC subset differentiation |
↓ Aortic CD103⁺ cDC1 → Altered T-cell accumulation/differentiation (↓ Th1/Tfh, ↑ Treg) |
[226] |
|
CCR2 |
Chemokine receptor for monocyte/DC migration |
• DCs: ↓ MHC-II/CD40 expression • Lymph nodes: ↓ IFN-γ⁺ cells |
|
|
CCR7 |
Key receptor for DC migration to lymph nodes |
Blocks DC trafficking to LNs → Inhibits adaptive immunity initiation |
[234] |
|
Clec9a |
cDC1 receptor for dead-cell antigens (DNGR-1) |
Impaired cDC1-mediated CD4⁺ T-cell regulation → ↑ Systemic IL-10 (atheroprotective) |
[146] |
|
Clec4a4 |
Pro-inflammatory CLR on cDC2s |
↓ Aortic sinus plaque/necrotic core ↑ Lipid profile improvement |
[235] |
|
CD40 |
Co-stimulatory molecule for DC-T cell interaction |
↓ Disease progression: Impaired DC-mediated T-cell activation → ↓ IFN-γ⁺ Th1 cells, ↑ IL-10⁺ Treg, reduced plaque necrosis |
[134] |
|
CD11c-DTR |
Diphtheria toxin receptor under CD11c promoter |
↑ Lesion severity: DT-mediated ablation of CD11c⁺ cells (DCs + macrophages) → ↑ Plasma cholesterol, ↓ Treg, ↑ Inflammatory cytokines |
[98] |
Notes: ↑: Increase; ↓: Decrease.
Table 3. Commonly used Cre mouse models for DC research.
|
Model |
Target Cells |
Specificity |
Application |
References |
|---|---|---|---|---|
|
CD11c-Cre |
cDCs, pDCs, macrophages |
Moderate |
DC/macrophage depletion studies Hypercholesterolemia mechanisms Pan-DC functional screening |
|
|
Zbtb46-Cre |
cDC1, cDC2 |
High |
cDC-specific gene ablation T-cell priming in plaques Plaque inflammation regulation |
[239] |
|
Clec9a-Cre |
cDC1 (Clec9a+) |
Very high |
Dead-cell antigen cross-presentation IL-10/Treg axis regulation Necrotic core dynamics |
[240] |
|
Xcr1-Cre |
cDC1 (Xcr1+) |
Very high |
cDC1 migration tracking CD8⁺ T-cell activation Batf3-dependent pathway analysis |
[241] |
Translating insights from mouse models to human diseases requires a precise understanding of DC heterogeneity and spatial positioning in human plaques. Emerging scRNA-seq and spatial transcriptomic datasets from human atherosclerotic arteries are now validating and refining these concepts [242,243]. These studies consistently identify human orthologs of mouse DC subsets and reveal their distinct transcriptional states associated with inflammation, lipid handling, and interferon response. Spatial omics further maps these DCs within the plaque microenvironment, positioning DCs in close proximity to T cells in areas of neovascularization and the shoulder region, thus directly visualizing the “immunological synapse” in situ [244,245]. This interaction network is pivotal for local T cell activation and polarization. Therefore, integrating functional genetics from mouse models with human multi-omics data provides a powerful framework for identifying novel therapeutic targets. Future strategies may aim to selectively inhibit pro-inflammatory DC subsets while leveraging regulatory DC functions to promote immune tolerance in atherosclerosis.
7. Remaining Questions and Future Directions
Although numerous studies have established the crucial role of DCs in atherosclerosis development, a deeper mechanistic understanding of DC heterogeneity and function within atherosclerotic lesions remains essential for designing effective targeted therapies. Key unresolved questions include: spatial distribution, functional duality, and ontogenetic pathways. Specifically, it remains unclear whether all DC subpopulations identified in secondary lymphoid organs reside in the arterial intima or adventitia; DCs’ capacity to drive disease progression via inflammation/immune activation, or confer protection through immune tolerance [246]; and the origins of vascular DCs, including recruitment of circulating precursors versus local proliferation, and factors regulating their plaque abundance.
In particular, several critical mechanistic questions highlighted by recent research deserve further investigation: While macrophages are the primary foam cells in atherosclerotic plaques, emerging evidence suggests that DCs also contribute to lipid accumulation and foam cell formation. The extent to which different DC subsets act as foam cell precursors, the specific receptors and metabolic pathways involved in DC lipid handling, and the functional consequences of lipid loading on DC immunogenicity or tolerance remain poorly defined. Second, the crosstalk between DCs and stromal cells within the plaque microenvironment remains poorly understood. Elucidating the bidirectional signals that dictate whether these interactions promote inflammation, fibrosis, or resolution is crucial for understanding plaque stability. Third, although migratory pathways for DC subsets have been delineated in other contexts, their relevance in atherosclerosis is underexplored. Defining the subset-specific routes and chemokine cues that govern DC recruitment to plaques, their navigation within the lesion, and their egress to draining lymph nodes will be vital for developing strategies to modulate local and systemic immune responses.
The complexity of DC roles in atherosclerosis manifests in their ability to mature and accumulate with T/B cells in plaques while simultaneously migrating to lymphoid organs. Resolving DC subset-specific migration patterns and precursor dynamics during atherogenesis is critical for identifying regulators of plaque DC accumulation, balancing their pro-inflammatory and tolerogenic functions, and developing immunotherapeutic strategies.
Furthermore, targeting DC recruitment, maturation, or functional polarization represents a promising frontier for modulating immune-driven vascular remodeling. Success requires balancing DC pro-inflammatory and tolerogenic functions and developing subset-specific interventions.
Acknowledgments
We thank all the members of GRIS in Xinxiang Medical University.
Author Contributions
Conceptualization, T.L. and Y.L.; Methodology, T.L.; Software, T.L.; Validation, L.L.; Investigation, J.Q. and D.L.; Writing—Original Draft Preparation, T.L.; Writing—Review & Editing, L.Z. and Y.L.; Visualization, T.L.; Funding Acquisition, Y.L. and T.L.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed in support of this review. All data discussed or cited are available from the original publications provided in the reference list.
Funding
This work was supported by National Natural Science Foundation of China (82301972), Science and Technology Department of Henan Province (242102310030) and 111 program (No. D20036).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
Libby P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar]
-
Ross R. Atherosclerosis--An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar]
-
Gu HF, Tang CK, Yang YZ. Psychological stress, immune response, and atherosclerosis. Atherosclerosis 2012, 223, 69–77. [Google Scholar]
-
Fernandes das Neves M, Batuca JR, Delgado Alves J. The role of high-density lipoprotein in the regulation of the immune response: Implications for atherosclerosis and autoimmunity. Immunology 2021, 164, 231–241. [Google Scholar]
-
Gistera A, Hansson GK. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar]
-
Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar]
-
Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, et al. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 243–250. [Google Scholar]
-
Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 2006, 203, 2073–2083. [Google Scholar]
-
Bobryshev YV, Lord RS. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of Vascular Dendritic Cells in athero-resistant and athero-prone areas of the normal aorta. Arch. Histol. Cytol. 1995, 58, 307–322. [Google Scholar]
-
Bobryshev YV, Lord RS. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc. Res. 1995, 29, 689–696. [Google Scholar]
-
Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2386–2391. [Google Scholar]
-
Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 2011, 35, 819–831. [Google Scholar]
-
Niessner A, Weyand CM. Dendritic cells in atherosclerotic disease. Clin. Immunol. 2010, 134, 25–32. [Google Scholar]
-
Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011, 32, 540–547. [Google Scholar]
-
Christ A, Temmerman L, Legein B, Daemen MJ, Biessen EA. Dendritic cells in cardiovascular diseases: epiphenomenon, contributor, or therapeutic opportunity. Circulation 2013, 128, 2603–2613. [Google Scholar]
-
Dieterlen MT, John K, Reichenspurner H, Mohr FW, Barten MJ. Dendritic Cells and Their Role in Cardiovascular Diseases: A View on Human Studies. J. Immunol. Res. 2016, 2016, 5946807. [Google Scholar]
-
Zhang Y, Zhang C. Role of dendritic cells in cardiovascular diseases. World J. Cardiol. 2010, 2, 357–364. [Google Scholar]
-
Bobryshev YV, Taksir T, Lord RS, Freeman MW. Evidence that dendritic cells infiltrate atherosclerotic lesions in apolipoprotein E-deficient mice. Histol. Histopathol. 2001, 16, 801–808. [Google Scholar]
-
Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin. Immunopathol. 2009, 31, 35–47. [Google Scholar]
-
Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 2007, 100, 884–893. [Google Scholar]
-
Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 2009, 206, 497–505. [Google Scholar]
-
Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin beta Receptors. Immunity 2015, 42, 1100–1115. [Google Scholar]
-
Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar]
-
Manthey HD, Zernecke A. Dendritic cells in atherosclerosis: Functions in immune regulation and beyond. Thromb. Haemost. 2011, 106, 772–778. [Google Scholar]
-
Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar]
-
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar]
-
Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007, 8, 1207–1216. [Google Scholar]
-
Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006, 211, 609–618. [Google Scholar]
-
Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J. Exp. Med. 2009, 206, 2141–2149. [Google Scholar]
-
Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 2010, 106, 383–390. [Google Scholar]
-
Zernecke A. Dendritic cells in atherosclerosis: Evidence in mice and humans. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 763–770. [Google Scholar]
-
Jin M, Fang J, Wang JJ, Shao X, Xu SW, Liu PQ, et al. Regulation of toll-like receptor (TLR) signaling pathways in atherosclerosis: From mechanisms to targeted therapeutics. Acta Pharmacol. Sin. 2023, 44, 2358–2375. [Google Scholar]
-
Bezhaeva T, Karper J, Quax PHA, de Vries MR. The Intriguing Role of TLR Accessory Molecules in Cardiovascular Health and Disease. Front. Cardiovasc. Med. 2022, 9, 820962. [Google Scholar]
-
Yang Q, Saaoud F, Lu Y, Pu Y, Xu K, Shao Y, et al. Innate immunity of vascular smooth muscle cells contributes to two-wave inflammation in atherosclerosis, twin-peak inflammation in aortic aneurysms and trans-differentiation potential into 25 cell types. Front. Immunol.2023, 14, 1348238. [Google Scholar]
-
Doodnauth SA, Grinstein S, Maxson ME. Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180147. [Google Scholar]
-
Appunni S, Rubens M, Ramamoorthy V, Anand V, Khandelwal M, Sharma A. Biglycan: an emerging small leucine-rich proteoglycan (SLRP) marker and its clinicopathological significance. Mol. Cell Biochem. 2021, 476, 3935–3950. [Google Scholar]
-
Jaen RI, Val-Blasco A, Prieto P, Gil-Fernandez M, Smani T, Lopez-Sendon JL, et al. Innate Immune Receptors, Key Actors in Cardiovascular Diseases. JACC Basic. Transl. Sci. 2020, 5, 735–749. [Google Scholar]
-
Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Investig. 2012, 122, 3114–3126. [Google Scholar]
-
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar]
-
Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab. Investig. 2010, 90, 970–984. [Google Scholar]
-
Legein B, Janssen EM, Theelen TL, Gijbels MJ, Walraven J, Klarquist JS, et al. Ablation of CD8alpha(+) dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice. Sci. Rep. 2015, 5, 15414. [Google Scholar]
-
Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar]
-
Reynolds G, Haniffa M. Human and Mouse Mononuclear Phagocyte Networks: A Tale of Two Species? Front. Immunol. 2015, 6, 330. [Google Scholar]
-
Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008, 9, R17. [Google Scholar]
-
Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, et al. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis 2004, 176, 101–110. [Google Scholar]
-
Bodder J, Zahan T, van Slooten R, Schreibelt G, de Vries IJM, Florez-Grau G. Harnessing the cDC1-NK Cross-Talk in the Tumor Microenvironment to Battle Cancer. Front. Immunol. 2020, 11, 631713. [Google Scholar]
-
Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 2010, 207, 823–836. [Google Scholar]
-
Crozat K, Tamoutounour S, Vu Manh TP, Fossum E, Luche H, Ardouin L, et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8alpha+ type. J. Immunol. 2011, 187, 4411–4415. [Google Scholar]
-
Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 2012, 36, 646–657. [Google Scholar]
-
Galibert L, Diemer GS, Liu Z, Johnson RS, Smith JL, Walzer T, et al. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J. Biol. Chem. 2005, 280, 21955–21964. [Google Scholar]
-
Van der Borght K, Scott CL, Nindl V, Bouche A, Martens L, Sichien D, et al. Myocardial Infarction Primes Autoreactive T Cells through Activation of Dendritic Cells. Cell Rep. 2017, 18, 3005–3017. [Google Scholar]
-
Bauriedel G, Skowasch D, Welsch U, Luderitz B. Role of dendritic cells in specific atherosclerosis types. Eur. Heart J. 2006, 27, 116–117. [Google Scholar]
-
Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar]
-
Vitali C, Mingozzi F, Broggi A, Barresi S, Zolezzi F, Bayry J, et al. Migratory, and not lymphoid-resident, dendritic cells maintain peripheral self-tolerance and prevent autoimmunity via induction of iTreg cells. Blood 2012, 120, 1237–1245. [Google Scholar]
-
Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012, 209, 653–660. [Google Scholar]
-
Mani V, Bromley SK, Aijo T, Mora-Buch R, Carrizosa E, Warner RD, et al. Migratory DCs activate TGF-beta to precondition naive CD8(+) T cells for tissue-resident memory fate. Science 2019, 366, eaav5728. [Google Scholar]
-
Rodriguez-Pla A, Patel P, Maecker HT, Rossello-Urgell J, Baldwin N, Bennett L, et al. IFN priming is necessary but not sufficient to turn on a migratory dendritic cell program in lupus monocytes. J. Immunol. 2014, 192, 5586–5598. [Google Scholar]
-
Gierlich P, Lex V, Technau A, Keupp A, Morper L, Glunz A, et al. Prostaglandin E(2) in a TLR3- and 7/8-agonist-based DC maturation cocktail generates mature, cytokine-producing, migratory DCs but impairs antigen cross-presentation to CD8(+) T cells. Cancer Immunol. Immunother. 2020, 69, 1029–1042. [Google Scholar]
-
Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 2009, 206, 359–370. [Google Scholar]
-
David R. Dendritic cells: The true face of migratory DCs. Nat. Rev. Immunol. 2014, 14, 649. [Google Scholar]
-
Ibrahim MK, Barnes JL, Osorio EY, Anstead GM, Jimenez F, Osterholzer JJ, et al. Deficiency of lymph node-resident dendritic cells (DCs) and dysregulation of DC chemoattractants in a malnourished mouse model of Leishmania donovani infection. Infect. Immun. 2014, 82, 3098–3112. [Google Scholar]
-
Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004, 25, 655–658. [Google Scholar]
-
Lee SW, Lee H, Lee KW, Kim MJ, Kang SW, Lee YJ, et al. CD8alpha+ dendritic cells potentiate antitumor and immune activities against murine ovarian cancers. Sci. Rep. 2023, 13, 98. [Google Scholar]
-
Shin JY, Wang CY, Lin CC, Chu CL. A recently described type 2 conventional dendritic cell (cDC2) subset mediates inflammation. Cell Mol. Immunol. 2020, 17, 1215–1217. [Google Scholar]
-
Hasegawa H, Matsumoto T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar]
-
Osorio F, Fuentes C, Lopez MN, Salazar-Onfray F, Gonzalez FE. Role of Dendritic Cells in the Induction of Lymphocyte Tolerance. Front. Immunol. 2015, 6, 535. [Google Scholar]
-
Fu C, Jiang A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar]
-
Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ. Res. 2011, 109, 1387–1395. [Google Scholar]
-
Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005, 434, 772–777. [Google Scholar]
-
Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J. Immunol. 2003, 170, 1131–1135. [Google Scholar]
-
Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity 2010, 33, 905–916. [Google Scholar]
-
Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar]
-
Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood 2006, 107, 2474–2476. [Google Scholar]
-
Ferrero I, Held W, Wilson A, Tacchini-Cottier F, Radtke F, MacDonald HR. Mouse CD11c(+) B220(+) Gr1(+) plasmacytoid dendritic cells develop independently of the T-cell lineage. Blood 2002, 100, 2852–2857. [Google Scholar]
-
Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997, 185, 1101–1111. [Google Scholar]
-
Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 2001, 194, 1823–1834. [Google Scholar]
-
Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 2013, 39, 925–938. [Google Scholar]
-
Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013, 34, 440–445. [Google Scholar]
-
McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E, et al. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity 2014, 41, 465–477. [Google Scholar]
-
Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19, 59–70. [Google Scholar]
-
Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar]
-
Dopheide JF, Sester U, Schlitt A, Horstick G, Rupprecht HJ, Munzel T, et al. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of co-stimulatory molecules CD40, CD80 and CD86 in vitro. Coron. Artery Dis. 2007, 18, 523–531. [Google Scholar]
-
Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 1999, 11, 753–761. [Google Scholar]
-
Heger L, Hofer TP, Bigley V, de Vries IJM, Dalod M, Dudziak D, et al. Subsets of CD1c(+) DCs: Dendritic Cell Versus Monocyte Lineage. Front. Immunol. 2020, 11, 559166. [Google Scholar]
-
Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 1999, 94, 3633–3643. [Google Scholar]
-
Menezes S, Melandri D, Anselmi G, Perchet T, Loschko J, Dubrot J, et al. The Heterogeneity of Ly6C(hi) Monocytes Controls Their Differentiation into iNOS(+) Macrophages or Monocyte-Derived Dendritic Cells. Immunity 2016, 45, 1205–1218. [Google Scholar]
-
Deng H, Sun Y, Zeng W, Li H, Guo M, Yang L, et al. New Classification of Macrophages in Plaques: a Revolution. Curr. Atheroscler. Rep. 2020, 22, 31. [Google Scholar]
-
Bosteels C, Fierens K, De Prijck S, Van Moorleghem J, Vanheerswynghels M, De Wolf C, et al. CCR2- and Flt3-Dependent Inflammatory Conventional Type 2 Dendritic Cells Are Necessary for the Induction of Adaptive Immunity by the Human Vaccine Adjuvant System AS01. Front. Immunol. 2020, 11, 606805. [Google Scholar]
-
Pereira da Costa M, Minutti CM, Piot C, Giampazolias E, Cardoso A, Cabeza-Cabrerizo M, et al. Interplay between CXCR4 and CCR2 regulates bone marrow exit of dendritic cell progenitors. Cell Rep. 2023, 42, 112881. [Google Scholar]
-
Cabeza-Cabrerizo M, Minutti CM, da Costa MP, Cardoso A, Jenkins RP, Kulikauskaite J, et al. Recruitment of dendritic cell progenitors to foci of influenza A virus infection sustains immunity. Sci. Immunol. 2021, 6, eabi9331. [Google Scholar]
-
Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar]
-
Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004, 110, 3493–3500. [Google Scholar]
-
Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, et al. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation 2005, 112, 1054–1062. [Google Scholar]
-
Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: Related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007, 25, 221–242. [Google Scholar]
-
Galan M, Fernandez-Mendez L, Nunez V, Femenia-Muina M, Figuera-Belmonte P, Moya-Ruiz E, et al. cDC1s Promote Atherosclerosis via Local Immunity and Are Targetable for Therapy. Circ. Res. 2025, 137, 400–416. [Google Scholar]
-
Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015, 42, 916–928. [Google Scholar]
-
Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar]
-
Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, et al. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 2009, 119, 2367–2375. [Google Scholar]
-
Yin X, Chen S, Eisenbarth SC. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar]
-
Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol. Cell Biol. 2010, 88, 257–268. [Google Scholar]
-
Gong F, Liu Z, Liu J, Zhou P, Liu Y, Lu X. The paradoxical role of IL-17 in atherosclerosis. Cell Immunol. 2015, 297, 33–39. [Google Scholar]
-
Talepoor AG, Fouladseresht H, Khosropanah S, Doroudchi M. Immune-Inflammation in Atherosclerosis: A New Twist in an Old Tale. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 525–545. [Google Scholar]
-
Pastrana JL, Sha X, Virtue A, Mai J, Cueto R, Lee IA, et al. Regulatory T cells and Atherosclerosis. J. Clin. Exp. Cardiolog. 2012, 2012 (Suppl. S12), 2. [Google Scholar]
-
Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, et al. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2569–2579. [Google Scholar]
-
Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012, 125, 1673–1683. [Google Scholar]
-
Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010, 12, 142–153. [Google Scholar]
-
Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 2006, 114, 2482–2489. [Google Scholar]
-
Doring Y, Zernecke A. Plasmacytoid dendritic cells in atherosclerosis. Front. Physiol. 2012, 3, 230. [Google Scholar]
-
Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar]
-
Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar]
-
Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 2008, 319, 198–202. [Google Scholar]
-
Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar]
-
Fang Z, Deng Q, Hu H, Wang X, Sun X, Ge X, et al. Characteristics of immunogenic and tolerogenic dendritic cells within the arterial wall in atherosclerosis and in vitro. Int. J. Clin. Exp. Med. 2014, 7, 4846–4856. [Google Scholar]
-
Sasaki N, Yamashita T, Kasahara K, Takeda M, Hirata K. Regulatory T cells and tolerogenic dendritic cells as critical immune modulators in atherogenesis. Curr. Pharm. Des. 2015, 21, 1107–1117. [Google Scholar]
-
Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1463–1470. [Google Scholar]
-
Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ. Res. 2008, 103, 965–973. [Google Scholar]
-
Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. USA 2004, 101, 11779–11784. [Google Scholar]
-
Bobryshev YV, Lord RS. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J. Histochem. Cytochem. 2005, 53, 781–785. [Google Scholar]
-
Cybulsky MI, Jongstra-Bilen J. Resident intimal dendritic cells and the initiation of atherosclerosis. Curr. Opin. Lipidol. 2010, 21, 397–403. [Google Scholar]
-
Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011, 35, 323–335. [Google Scholar]
-
Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, et al. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis 2009, 205, 442–450. [Google Scholar]
-
Alderman CJ, Bunyard PR, Chain BM, Foreman JC, Leake DS, Katz DR. Effects of oxidised low density lipoprotein on dendritic cells: A possible immunoregulatory component of the atherogenic micro-environment? Cardiovasc. Res. 2002, 55, 806–819. [Google Scholar]
-
Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar]
-
Sun J, Hartvigsen K, Chou MY, Zhang Y, Sukhova GK, Zhang J, et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation 2010, 122, 808–820. [Google Scholar]
-
Lievens D, Habets KL, Robertson AK, Laouar Y, Winkels H, Rademakers T, et al. Abrogated transforming growth factor beta receptor II (TGFbetaRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur. Heart J. 2013, 34, 3717–3727. [Google Scholar]
-
Weber C, Meiler S, Doring Y, Koch M, Drechsler M, Megens RT, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Investig. 2011, 121, 2898–2910. [Google Scholar]
-
Ye Y, Yang X, Zhao X, Chen L, Xie H, Zeng Y, et al. Serum chemokine CCL17/thymus activation and regulated chemokine is correlated with coronary artery diseases. Atherosclerosis 2015, 238, 365–369. [Google Scholar]
-
Han SF, Liu P, Zhang W, Bu L, Shen M, Li H, et al. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin. Immunol. 2007, 124, 90–97. [Google Scholar]
-
Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3781–3786. [Google Scholar]
-
Xu X, Mo L, Liao Y, Zhang KS, Zhang H, Liu L, et al. An association between elevated telomerase reverse transcriptase expression and the immune tolerance disruption of dendritic cells. Cell Commun. Signal 2024, 22, 284. [Google Scholar]
-
Wolf D, Gerhardt T, Winkels H, Michel NA, Pramod AB, Ghosheh Y, et al. Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B(100)-Reactive CD4(+) T-Regulatory Cells. Circulation 2020, 142, 1279–1293. [Google Scholar]
-
van Es T, van Puijvelde GH, Foks AC, Habets KL, Bot I, Gilboa E, et al. Vaccination against Foxp3(+) regulatory T cells aggravates atherosclerosis. Atherosclerosis 2010, 209, 74–80. [Google Scholar]
-
Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation 2011, 123, 1083–1091. [Google Scholar]
-
Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, et al. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc. Res. 2010, 85, 622–630. [Google Scholar]
-
Bobryshev YV, Watanabe T. Ultrastructural evidence for association of vascular dendritic cells with T-lymphocytes and with B-cells in human atherosclerosis. J. Submicrosc. Cytol. Pathol. 1997, 29, 209–221. [Google Scholar]
-
Woszczek G, Chen LY, Nagineni S, Shelhamer JH. IL-10 inhibits cysteinyl leukotriene-induced activation of human monocytes and monocyte-derived dendritic cells. J. Immunol. 2008, 180, 7597–7603. [Google Scholar]
-
Shabgah AG, Shariati-Sarabi Z, Tavakkol-Afshari J, Mohammadi M. The role of BAFF and APRIL in rheumatoid arthritis. J. Cell Physiol. 2019, 234, 17050–17063. [Google Scholar]
-
Sun L, Zhang W, Zhao L, Zhao Y, Wang F, Lew AM, et al. Self-Tolerance of Vascular Tissues Is Broken Down by Vascular Dendritic Cells in Response to Systemic Inflammation to Initiate Regional Autoinflammation. Front. Immunol. 2022, 13, 823853. [Google Scholar]
-
Hilgendorf I, Theurl I, Gerhardt LM, Robbins CS, Weber GF, Gonen A, et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 2014, 129, 1677–1687. [Google Scholar]
-
Doring Y, van der Vorst EPC, Weber C. Targeting immune cell recruitment in atherosclerosis. Nat. Rev. Cardiol. 2024, 21, 824–840. [Google Scholar]
-
Weis M, Schlichting CL, Engleman EG, Cooke JP. Endothelial determinants of dendritic cell adhesion and migration: new implications for vascular diseases. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1817–1823. [Google Scholar]
-
Breslow JL. Genetic differences in endothelial cells may determine atherosclerosis susceptibility. Circulation 2000, 102, 5–6. [Google Scholar]
-
Bosseboeuf E, Raimondi C. Signalling, Metabolic Pathways and Iron Homeostasis in Endothelial Cells in Health, Atherosclerosis and Alzheimer’s Disease. Cells 2020, 9, 2055. [Google Scholar]
-
Li S, Zhu WG, Yan H, Fan FY, Sun PY, Zhu JH. Homocysteine at pathophysiological concentrations enhances binding of dendritic cells to endothelial cells mediated by DC-SIGN. Int. Immunopharmacol. 2007, 7, 1241–1250. [Google Scholar]
-
Broder A, Chan JJ, Putterman C. Dendritic cells: an important link between antiphospholipid antibodies, endothelial dysfunction, and atherosclerosis in autoimmune and non-autoimmune diseases. Clin. Immunol. 2013, 146, 197–206. [Google Scholar]
-
de la Rosa G, Longo N, Rodriguez-Fernandez JL, Puig-Kroger A, Pineda A, Corbi AL, et al. Migration of human blood dendritic cells across endothelial cell monolayers: Adhesion molecules and chemokines involved in subset-specific transmigration. J. Leukoc. Biol. 2003, 73, 639–649. [Google Scholar]
-
Del Prete A, Locati M, Otero K, Riboldi E, Mantovani A, Vecchi A, et al. Migration of dendritic cells across blood and lymphatic endothelial barriers. Thromb. Haemost. 2006, 95, 22–28. [Google Scholar]
-
Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, et al.The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 2001, 193, 1105–1112. [Google Scholar]
-
Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011, 118, 205–215. [Google Scholar]
-
Affandi AJ, Carvalheiro T, Radstake T, Marut W. Dendritic cells in systemic sclerosis: Advances from human and mice studies. Immunol. Lett. 2018, 195, 18–29. [Google Scholar]
-
Miano JM, Fisher EA, Majesky MW. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar]
-
Kloc M, Kubiak JZ, Ghobrial RM. Macrophage-, Dendritic-, Smooth Muscle-, Endothelium-, and Stem Cells-Derived Foam Cells in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 14154. [Google Scholar]
-
Paccosi S, Musilli C, Caporale R, Gelli AM, Guasti D, Clemente AM, et al. Stimulatory interactions between human coronary smooth muscle cells and dendritic cells. PLoS ONE 2014, 9, e99652. [Google Scholar]
-
Clement M, Raffort J, Lareyre F, Tsiantoulas D, Newland S, Lu Y, et al. Impaired Autophagy in CD11b(+) Dendritic Cells Expands CD4(+) Regulatory T Cells and Limits Atherosclerosis in Mice. Circ. Res. 2019, 125, 1019–1034. [Google Scholar]
-
Bobryshev YV, Ikezawa T, Watanabe T. Formation of Birbeck granule-like structures in vascular dendritic cells in human atherosclerotic aorta. Lag-antibody to epidermal Langerhans cells recognizes cells in the aortic wall. Atherosclerosis 1997, 133, 193–202. [Google Scholar]
-
Sasaki N, Yamashita T, Kasahara K, Fukunaga A, Yamaguchi T, Emoto T, et al. UVB Exposure Prevents Atherosclerosis by Regulating Immunoinflammatory Responses. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 66–74. [Google Scholar]
-
Matsuo K, Yoshie O, Kitahata K, Kamei M, Hara Y, Nakayama T. Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers 2021, 13, 2495. [Google Scholar]
-
Ding J, Zheng Y, Wang G, Zheng J, Chai D. The performance and perspectives of dendritic cell vaccines modified by immune checkpoint inhibitors or stimulants. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188763. [Google Scholar]
-
Hotchkiss KM, Batich KA, Mohan A, Rahman R, Piantadosi S, Khasraw M. Dendritic cell vaccine trials in gliomas: Untangling the lines. Neuro Oncol. 2023, 25, 1752–1762. [Google Scholar]
-
Schinnerling K, Soto L, Garcia-Gonzalez P, Catalan D, Aguillon JC. Skewing dendritic cell differentiation towards a tolerogenic state for recovery of tolerance in rheumatoid arthritis. Autoimmun. Rev. 2015, 14, 517–527. [Google Scholar]
-
Gross CC, Wiendl H. Dendritic cell vaccination in autoimmune disease. Curr. Opin. Rheumatol. 2013, 25, 268–274. [Google Scholar]
-
Cifuentes-Rius A, Desai A, Yuen D, Johnston APR, Voelcker NH. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar]
-
Ide F, Nakamura T, Tomizawa M, Kawana-Tachikawa A, Odawara T, Hosoya N, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J. Med. Virol. 2006, 78, 711–718. [Google Scholar]
-
Akbar SM, Furukawa S, Hasebe A, Horiike N, Michitaka K, Onji M. Production and efficacy of a dendritic cell-based therapeutic vaccine for murine chronic hepatitis B virus carrierer. Int. J. Mol. Med. 2004, 14, 295–299. [Google Scholar]
-
Hong B, Lee SH, Song XT, Jones L, Machida K, Huang XF, et al. A super TLR agonist to improve efficacy of dendritic cell vaccine in induction of anti-HCV immunity. PLoS ONE 2012, 7, e48614. [Google Scholar]
-
Whiteside TL, Piazza P, Reiter A, Stanson J, Connolly NC, Rinaldo CR, et al. Production of a dendritic cell-based vaccine containing inactivated autologous virus for therapy of patients with chronic human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 2009, 16, 233–240. [Google Scholar]
-
Oshiro TM, de Almeida A, da Silva Duarte AJ. Dendritic cell immunotherapy for HIV infection: From theory to reality. Immunotherapy 2009, 1, 1039–1051. [Google Scholar]
-
Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D. Update on Dendritic Cell-Induced Immunological and Clinical Tolerance. Front. Immunol. 2017, 8, 1514. [Google Scholar]
-
Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar]
-
Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar]
-
Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 95–100. [Google Scholar]
-
Inoue T, Uchida T, Kamishirado H, Takayanagi K, Hayashi T, Morooka S. Clinical significance of antibody against oxidized low density lipoprotein in patients with atherosclerotic coronary artery disease. J. Am. Coll. Cardiol. 2001, 37, 775–779. [Google Scholar]
-
Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler. Thromb. 1994, 14, 32–40. [Google Scholar]
-
Frodermann V, van Puijvelde GH, Wierts L, Lagraauw HM, Foks AC, van Santbrink PJ, et al. Oxidized low-density lipoprotein-induced apoptotic dendritic cells as a novel therapy for atherosclerosis. J. Immunol. 2015, 194, 2208–2218. [Google Scholar]
-
Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension 2000, 36, 303–307. [Google Scholar]
-
Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, et al. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler. Thromb. 1992, 12, 789–799. [Google Scholar]
-
Rahman M, Steuer J, Gillgren P, Hayderi A, Liu A, Frostegard J. Induction of Dendritic Cell-Mediated Activation of T Cells From Atherosclerotic Plaques by Human Heat Shock Protein 60. J. Am. Heart Assoc. 2017, 6, e006778. [Google Scholar]
-
Mundkur L, Mukhopadhyay R, Samson S, Varma M, Kale D, Chen D, et al. Mucosal tolerance to a combination of ApoB and HSP60 peptides controls plaque progression and stabilizes vulnerable plaque in Apob(tm2Sgy)Ldlr(tm1Her)/J mice. PLoS ONE 2013, 8, e58364. [Google Scholar]
-
Lu X, Chen D, Endresz V, Xia M, Faludi I, Burian K, et al. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis 2010, 212, 472–480. [Google Scholar]
-
Chyu KY, Zhao X, Zhou J, Dimayuga PC, Lio NW, Cercek B, et al. Immunization using ApoB-100 peptide-linked nanoparticles reduces atherosclerosis. JCI Insight 2022, 7, e149741. [Google Scholar]
-
George J, Harats D, Gilburd B, Afek A, Levy Y, Schneiderman J, et al. Immunolocalization of beta2-glycoprotein I (apolipoprotein H) to human atherosclerotic plaques: potential implications for lesion progression. Circulation 1999, 99, 2227–2230. [Google Scholar]
-
George J, Harats D, Gilburd B, Afek A, Shaish A, Kopolovic J, et al. Adoptive transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation 2000, 102, 1822–1827. [Google Scholar]
-
Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol. Rev. 2011, 241, 241–259. [Google Scholar]
-
Liu A, Ming JY, Fiskesund R, Ninio E, Karabina SA, Bergmark C, et al. Induction of dendritic cell-mediated T-cell activation by modified but not native low-density lipoprotein in humans and inhibition by annexin a5: involvement of heat shock proteins. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 197–205. [Google Scholar]
-
Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, et al. A critical function of Th17 pro-inflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 2010, 185, 5820–5827. [Google Scholar]
-
Zhang A, Fu J, Ning B, Li D, Sun N, Wei W, et al. Tolerogenic dendritic cells generated with IL-10/TGFbeta1 relieve immune thrombocytopenia in mice. Thromb. Res. 2013, 132, 63–68. [Google Scholar]
-
Yoshimatsu H, Kataoka K, Fujihashi K, Miyake T, Ono Y. A nasal double DNA adjuvant system induces atheroprotective IgM antibodies via dendritic cell-B-1a B cell interactions. Vaccine 2022, 40, 1116–1127. [Google Scholar]
-
Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, et al. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: New implications for atherosclerosis. Atherosclerosis 2004, 172, 85–93. [Google Scholar]
-
Frostegard J. The role of PCSK9 in inflammation, immunity, and autoimmune diseases. Expert. Rev. Clin. Immunol. 2022, 18, 67–74. [Google Scholar]
-
Kofler S, Schlichting C, Jankl S, Nickel T, Weis M. Dual mode of HMG-CoA reductase inhibition on dendritic cell invasion. Atherosclerosis 2008, 197, 105–110. [Google Scholar]
-
Yilmaz A, Reiss C, Weng A, Cicha I, Stumpf C, Steinkasserer A, et al. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J. Leukoc. Biol. 2006, 79, 529–538. [Google Scholar]
-
Luo Y, Liang C, Xu C, Jia Q, Huang D, Chen L, et al. Ciglitazone inhibits oxidized-low density lipoprotein induced immune maturation of dendritic cells. J. Cardiovasc. Pharmacol. 2004, 44, 381–385. [Google Scholar]
-
Shi HY, Ge JB, Fang WY, Yao K, Sun AJ, Huang RC, et al. Peroxisome proliferator-activated receptor alpha agonist attenuates oxidized-low density lipoprotein induced immune maturation of human monocyte-derived dendritic cells. Chin. Med. J. 2008, 121, 1747–1750. [Google Scholar]
-
Su W, Sun A, Xu D, Zhang H, Yang L, Yuan L, et al. Tongxinluo inhibits oxidized low-density lipoprotein-induced maturation of human dendritic cells via activating peroxisome proliferator-activated receptor gamma pathway. J. Cardiovasc. Pharmacol. 2010, 56, 177–183. [Google Scholar]
-
Liu H, Wang S, Sun A, Huang D, Wang W, Zhang C, et al. Danhong inhibits oxidized low-density lipoprotein-induced immune maturation of dentritic cells via a peroxisome proliferator activated receptor gamma-mediated pathway. J. Pharmacol. Sci. 2012, 119, 1–9. [Google Scholar]
-
Zhao J, Zhu H, Wang S, Ma X, Liu X, Wang C, et al. Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. Curr. Pharm. Des. 2013, 19, 5891–5896. [Google Scholar]
-
Jiao F, Varghese K, Wang S, Liu Y, Yu H, Booz GW, et al. Recent Insights Into the Protective Mechanisms of Paeoniflorin in Neurological, Cardiovascular, and Renal Diseases. J. Cardiovasc. Pharmacol. 2021, 77, 728–734. [Google Scholar]
-
Sochorova K, Budinsky V, Rozkova D, Tobiasova Z, Dusilova-Sulkova S, Spisek R, et al. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin. Immunol. 2009, 133, 69–77. [Google Scholar]
-
Tedgui A, Owens AP, 3rd, Mackman N. Nobel Prize in Physiology or Medicine: Toll-like receptors, dendritic cells, and their roles in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2767–2768. [Google Scholar]
-
Vellasamy DM, Lee SJ, Goh KW, Goh BH, Tang YQ, Ming LC, et al. Targeting Immune Senescence in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 13059. [Google Scholar]
-
Doherty TM, Fisher EA, Arditi M. TLR signaling and trapped vascular dendritic cells in the development of atherosclerosis. Trends Immunol. 2006, 27, 222–227. [Google Scholar]
-
Wang YK, Wang J, Hua F, Shen YL, Han L, You JY, et al. TREM-1 Modulates Dendritic Cells Maturation and Dendritic Cell-Mediated T-Cell Activation Induced by ox-LDL. Oxid. Med. Cell Longev. 2022, 2022, 3951686. [Google Scholar]
-
Rai V, Rao VH, Shao Z, Agrawal DK. Dendritic Cells Expressing Triggering Receptor Expressed on Myeloid Cells-1 Correlate with Plaque Stability in Symptomatic and Asymptomatic Patients with Carotid Stenosis. PLoS ONE 2016, 11, e0154802. [Google Scholar]
-
Sabatte J, Faigle W, Ceballos A, Morelle W, Rodriguez Rodrigues C, Remes Lenicov F, et al. Semen clusterin is a novel DC-SIGN ligand. J. Immunol. 2011, 187, 5299–5309. [Google Scholar]
-
Yang K, Liu X, Liu Y, Wang X, Cao L, Zhang X, et al. DC-SIGN and Toll-like receptor 4 mediate oxidized low-density lipoprotein-induced inflammatory responses in macrophages. Sci. Rep. 2017, 7, 3296. [Google Scholar]
-
Soilleux EJ, Morris LS, Trowsdale J, Coleman N, Boyle JJ. Human atherosclerotic plaques express DC-SIGN, a novel protein found on dendritic cells and macrophages. J. Pathol. 2002, 198, 511–516. [Google Scholar]
-
Wang L, Li D, Yang K, Hu Y, Zeng Q. Toll-like receptor-4 and mitogen-activated protein kinase signal system are involved in activation of dendritic cells in patients with acute coronary syndrome. Immunology 2008, 125, 122–130. [Google Scholar]
-
Liu T, Liu J, Lin Y, Que B, Chang C, Zhang J, et al. IL-37 inhibits the maturation of dendritic cells through the IL-1R8-TLR4-NF-kappaB pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1338–1349. [Google Scholar]
-
Nilsson J, Fredrikson GN, Bjorkbacka H, Chyu KY, Shah PK. Vaccines modulating lipoprotein autoimmunity as a possible future therapy for cardiovascular disease. J. Intern. Med. 2009, 266, 221–231. [Google Scholar]
-
Vallejo J, Cochain C, Zernecke A, Ley K. Heterogeneity of immune cells in human atherosclerosis revealed by scRNA-Seq. Cardiovasc. Res. 2021, 117, 2537–2543. [Google Scholar]
-
Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar]
-
Depuydt MAC, Prange KHM, Slenders L, Ord T, Elbersen D, Boltjes A, et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020, 127, 1437–1455. [Google Scholar]
-
Suzuki M, Yamamoto D, Suzuki T, Fujii M, Suzuki N, Fujishiro M, et al. High fat and high fructose diet induced intracranial atherosclerosis and enhanced vasoconstrictor responses in non-human primate. Life Sci. 2006, 80, 200–204. [Google Scholar]
-
Di Cataldo V, Debatisse J, Piraquive J, Geloen A, Grandin C, Verset M, et al. Cortical inflammation and brain signs of high-risk atherosclerosis in a non-human primate model. Brain Commun. 2021, 3, fcab064. [Google Scholar]
-
Paslawski R, Kowalczyk P, Paslawska U, Wiśniewski J, Dzięgiel P, Janiszewski A, et al. Analysis of the Model of Atherosclerosis Formation in Pig Hearts as a Result of Impaired Activity of DNA Repair Enzymes. Int. J. Mol. Sci. 2024, 25, 2282. [Google Scholar]
-
Hoogendoorn A, den Hoedt S, Hartman EMJ, Krabbendam-Peters I, Te Lintel Hekkert M, van der Zee L, et al. Variation in Coronary Atherosclerosis Severity Related to a Distinct LDL (Low-Density Lipoprotein) Profile: Findings From a Familial Hypercholesterolemia Pig Model. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2338–2352. [Google Scholar]
-
Baumgartner C, Brandl J, Munch G, Ungerer M. Rabbit models to study atherosclerosis and its complications—Transgenic vascular protein expression in vivo. Prog. Biophys. Mol. Biol. 2016, 121, 131–141. [Google Scholar]
-
Schlegel A. Zebrafish Models for Dyslipidemia and Atherosclerosis Research. Front. Endocrinol. 2016, 7, 159. [Google Scholar]
-
Fang L, Liu C, Miller YI. Zebrafish models of dyslipidemia: relevance to atherosclerosis and angiogenesis. Transl. Res. 2014, 163, 99–108. [Google Scholar]
-
Hasenstab D, Lea H, Hart CE, Lok S, Clowes AW. Tissue factor overexpression in rat arterial neointima models thrombosis and progression of advanced atherosclerosis. Circulation 2000, 101, 2651–2657. [Google Scholar]
-
Oppi S, Luscher TF, Stein S. Mouse Models for Atherosclerosis Research-Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar]
-
Getz GS, Reardon CA. Do the Apoe-/- and Ldlr-/- Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar]
-
Gil-Pulido J, Cochain C, Lippert MA, Schneider N, Butt E, Amezaga N, et al. Deletion of Batf3-dependent antigen-presenting cells does not affect atherosclerotic lesion formation in mice. PLoS ONE 2017, 12, e0181947. [Google Scholar]
-
Li Y, Liu X, Duan W, Tian H, Zhu G, He H, et al. Batf3-dependent CD8alpha(+) Dendritic Cells Aggravates Atherosclerosis via Th1 Cell Induction and Enhanced CCL5 Expression in Plaque Macrophages. EBioMedicine 2017, 18, 188–198. [Google Scholar]
-
Chauhan KS, Das A, Jaiswal H, Saha I, Kaushik M, Patel VK, et al. IRF8 and BATF3 interaction enhances the cDC1 specific Pfkfb3 gene expression. Cell Immunol. 2022, 371, 104468. [Google Scholar]
-
Clement M, Haddad Y, Raffort J, Lareyre F, Newland SA, Master L, et al. Deletion of IRF8 (Interferon Regulatory Factor 8)-Dependent Dendritic Cells Abrogates Proatherogenic Adaptive Immunity. Circ. Res. 2018, 122, 813–820. [Google Scholar]
-
Cueto FJ, Sancho D. The Flt3L/Flt3 Axis in Dendritic Cell Biology and Cancer Immunotherapy. Cancers 2021, 13, 1525. [Google Scholar]
-
Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009, 206, 3115–3130. [Google Scholar]
-
Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood 2009, 114, 835–843. [Google Scholar]
-
Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr., Broxmeyer HE, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 1997, 100, 2552–2561. [Google Scholar]
-
Cedile O, Jorgensen LO, Frank I, Wlodarczyk A, Owens T. The chemokine receptor CCR2 maintains plasmacytoid dendritic cell homeostasis. Immunol. Lett. 2017, 192, 72–78. [Google Scholar]
-
Chiu BC, Freeman CM, Stolberg VR, Hu JS, Zeibecoglou K, Lu B, et al. Impaired lung dendritic cell activation in CCR2 knockout mice. Am. J. Pathol. 2004, 165, 1199–1209. [Google Scholar]
-
Peters W, Dupuis M, Charo IF. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J. Immunol. 2000, 165, 7072–7077. [Google Scholar]
-
Wan W, Lionakis MS, Liu Q, Roffe E, Murphy PM. Genetic deletion of chemokine receptor Ccr7 exacerbates atherogenesis in ApoE-deficient mice. Cardiovasc. Res. 2013, 97, 580–588. [Google Scholar]
-
Britsch S, Langer H, Duerschmied D, Becher T. The Evolving Role of Dendritic Cells in Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 2450. [Google Scholar]
-
Sauter M, Sauter RJ, Nording H, Lin C, Olbrich M, Autenrieth S, et al. Apolipoprotein E derived from CD11c(+) cells ameliorates atherosclerosis. iScience 2022, 25, 103677. [Google Scholar]
-
Nieuwenhuizen NE, Kirstein F, Hoving JC, Brombacher F. House dust mite induced allergic airway disease is attenuated in CD11c(cre)IL-4Ralpha(-/l) degrees (x) mice. Sci. Rep. 2018, 8, 885. [Google Scholar]
-
Manouchehri N, Hussain RZ, Cravens PD, Doelger R, Greenberg BM, Okuda DT, et al. Limitations of cell-lineage-specific non-dynamic gene recombination in CD11c.Cre(+)ITGA4(fl/fl) mice. J. Neuroimmunol. 2020, 344, 577245. [Google Scholar]
-
Wu X, Briseno CG, Durai V, Albring JC, Haldar M, Bagadia P, et al. Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J. Exp. Med. 2016, 213, 2553–2565. [Google Scholar]
-
Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 2013, 154, 843–858. [Google Scholar]
-
Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 2019, 4, eaau8380. [Google Scholar]
-
Mazan-Mamczarz K, Tsitsipatis D, Childs BG, Carr AE, Dos Santos CR, Anerillas C, et al. Single-cell and spatial transcriptomics map senescent vascular cells in arterial remodeling during atherosclerosis in mice. Nat. Aging 2025, 5, 1528–1547. [Google Scholar]
-
Bleckwehl T, Babler A, Tebens M, Maryam S, Nyberg M, Bosteen M, et al. Encompassing view of spatial and single-cell RNA sequencing renews the role of the microvasculature in human atherosclerosis. Nat. Cardiovasc. Res. 2025, 4, 26–44. [Google Scholar]
-
Monaco C, McNamara CA, Slutter B, Foks AC, Bekiranov S, Mulder WJM, et al. Immunotherapy for atherosclerosis. Physiol. Rev. 2025, 105, 2141–2230. [Google Scholar]
-
Cetin E, Raby AC. Understanding Atherosclerotic Plaque Cellular Composition: Recent Advances Driven by Single Cell Omics. Cells 2025, 14, 770. [Google Scholar]
-
Bobryshev YV. Dendritic cells and their involvement in atherosclerosis. Curr. Opin. Lipidol. 2000, 11, 511–517. [Google Scholar]