Sitagliptin in Type 2 Diabetes Mellitus and Cardiovascular Disease: A Public Health and Health Equity Perspective
Received: 20 August 2025 Revised: 08 September 2025 Accepted: 28 September 2025 Published: 09 October 2025
© 2025 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
1.1. Type 2 Diabetes Mellitus and Cardiovascular Disease
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by elevated blood glucose levels due to insulin resistance and inadequate insulin secretion. It accounts for about 90–95% of diabetes cases and often coexists with other cardiometabolic conditions [1]. Cardiovascular disease (CVD), encompassing coronary artery disease, stroke, heart failure, and other vascular disorders, remains the leading cause of mortality in the U.S and globally worldwide [2,3]. Epidemiologic data indicate that individuals with diabetes have a two- to four-fold higher risk of myocardial infarction and stroke compared to those without diabetes [4]. According to the Centers for Disease Control and Prevention [5], an estimated 38.4 million people, or 11.6% of the U.S. population, live with diabetes. Importantly, individuals with diabetes are at markedly increased risk of CVD complications.
1.2. Disproportionate Burden in Underserved Populations
T2DM and CVD disproportionately affect underserved communities. Racial minority groups and people of lower socio-economic status have higher prevalence of T2DM, poorer control, and higher rates of complications. In the U.S., Black and Latino adults are significantly more likely to develop T2DM (60% and 17% higher risk, respectively, than White adults) and suffer its complications [6]. Lower-income individuals are 2 to 4 times more likely to develop diabetes and often struggle to maintain glycemic control [7]. Similar disparities exist in CVD; people in under-resourced communities experience higher rates of hypertension, ischemic heart disease, and heart failure, partly due to barriers in accessing preventive care. These inequities stem from social determinants like poverty, limited healthcare access, education gaps, and chronic stress [6]. Tackling diabetes and CVD, therefore, requires not only clinical management but also a health equity approach to ensure underserved populations benefit from advanced treatment modalities.
1.3. Sitagliptin: Safety, Use, and Access in Contemporary Practice
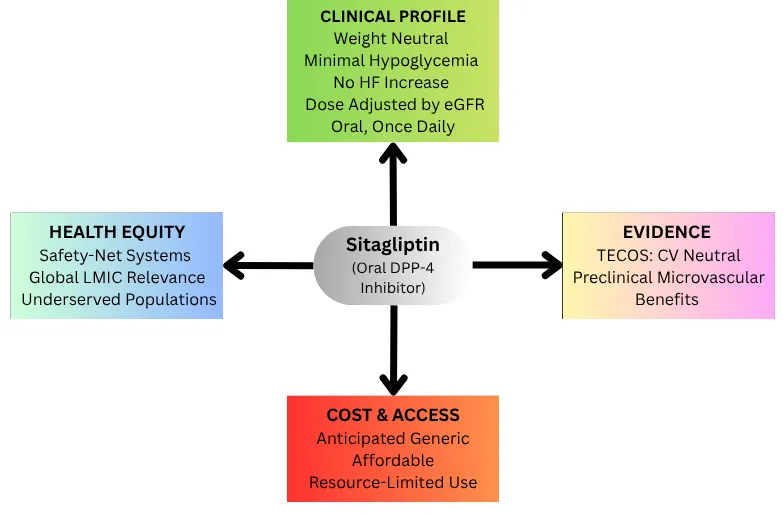
Sitagliptin was the first oral Dipeptidyl peptidase-4 (DPP-4) inhibitor approved for the treatment of T2DM in the United States in 2006 and has since become one of the most widely studied agents in its class [8,9]. Its clinical adoption was driven by a favorable safety profile [10,11]. As incretin-based therapies gained traction, sitagliptin became a practical oral option for patients who could not tolerate or afford injectable medications.
While newer agents like glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose co-transporter 2 (SGLT2) inhibitors have demonstrated cardiovascular and renal benefits, DPP-4 inhibitors including sitagliptin have consistently shown cardiovascular neutrality as evidenced by the TECOS trial [10]. This neutrality, meaning unchanged risk of major cardiovascular events, was sufficient for regulatory approval and clinical acceptance.
In some US states, including Illinois, an authorized generic version of sitagliptin is listed on the Medicaid Preferred Drug List, improving accessibility [12]. However, with no fully generic version yet available in the U.S., coverage remains inconsistent across states, and nationwide disparities persist due to variable formularies, prior authorization requirements, limited private insurance, and broader systemic inequities in healthcare [13].
This article addresses this main question: What is the logical and equitable place of sitagliptin in a therapeutic landscape dominated by organ-protective agents? We therefore proceed from mechanism to outcomes to implementation to specify when sitagliptin adds value for patients and health systems.
1.4. Pathophysiology Linking T2DM to CVD
The metabolic derangements in T2DM directly contribute to cardiovascular pathology. Chronically elevated blood glucose and insulin resistance set off a cascade of detrimental effects on blood vessels. Hyperglycemia and insulin resistance promote oxidative stress, inflammation, and dyslipidemia, accelerating atherosclerosis [14]. Insulin resistance is often accompanied by hypertension, an integral part of the “diabetic dysmetabolic syndrome” that worsens vascular injury. Excess glucose and free fatty acids trigger inflammatory signaling in adipose tissue and blood vessels; pro-inflammatory cytokines (e.g., TNF-α, IL-6) and adipokines promote fatty streak formation and unstable plaque in arteries. In essence, T2DM creates an environment of chronic inflammation and oxidative stress that fosters the development of coronary artery disease, peripheral arterial disease, and stroke over time. By the time diabetes is diagnosed, many patients already have subclinical atherosclerosis [15]. This helps explain why patients with diabetes are considered at elevated cardiovascular risk, prompting guidelines to emphasize aggressive management of blood pressure, LDL cholesterol, smoking cessation, and other cardiovascular risk factors, alongside glycemic control [16].
1.5. Diabetes and Vascular Function
Chronic hyperglycemia in diabetes promotes oxidative stress, inflammation, and the accumulation of advanced glycation end-products (AGEs), which impair endothelial nitric oxide synthase (eNOS) activity and reduce nitric oxide (NO) bioavailability, defining features of endothelial dysfunction [17,18]. This early vascular injury paves the way for macrovascular complications like atherosclerosis, heart attack, stroke, and peripheral artery disease, as well as microvascular damage, including retinopathy, nephropathy, and neuropathy. Endothelial dysfunction disrupts vascular tone, promotes inflammation, and accelerates atherogenesis. Preserving endothelial health is therefore essential to prevent these long-term outcomes. DPP‑4 inhibitors may offer vascular protection beyond glucose control, with studies showing anti‑inflammatory and antioxidant effects, improved endothelial function, and reduced urinary albumin excretion. Preclinical work further supports this: sitagliptin restored aortic endothelial relaxation, corrected dyslipidemia, and reactivated AMPK/eNOS signaling by downregulating the Creb5/lncRNA ENSMUST00000213271 pathway [19]. Because AMPK/eNOS is a critical axis for NO production and vascular tone, these findings suggest that sitagliptin may help prevent macro- and microvascular complications in diabetes by protecting endothelial function.
1.6. Cardiac Remodeling and Diabetic Cardiomyopathy
Beyond atherosclerotic disease, diabetes detrimentally affects the myocardium and can lead to heart failure independent of coronary artery disease. Persistently high glucose and insulin resistance provoke molecular changes in heart muscle cells, impaired insulin signaling in cardiomyocytes, mitochondrial dysfunction, and excessive accumulation of fatty deposits in the heart, all of which contribute to myocardial fibrosis and stiffness [20]. This clinical entity, termed diabetic cardiomyopathy, is characterized early on by left ventricular hypertrophy, interstitial fibrosis, and diastolic dysfunction (impaired relaxation). Patients may develop heart failure with preserved ejection fraction (HFpEF) as a result. Over time, if these processes continue unchecked, systolic function can also deteriorate, progressing to overt heart failure [21]. In diabetes, the heart is also subject to the renin-angiotensin-aldosterone system (RAAS) overactivation and sympathetic drive compounding remodeling [20]. Inflammatory cells infiltrate the myocardium and small vessels, while oxidative stress injures cardiac tissues. The key takeaway here is that people with T2DM are not only more prone to ischemic heart disease but also to cardiomyopathic changes that increase the risk of heart failure (even in the absence of prior myocardial infarction). This dual impact, accelerating atherosclerosis and causing direct myocardial injury, underlies the strong epidemiologic association between T2DM and virtually all forms of CVD.
1.7. Pharmacology of DPP-4 Inhibitors with a Focus on Sitagliptin
Sitagliptin is the prototype DPP-4 inhibitor, a class of oral antihyperglycemic agents that augment the action of incretin hormones, primarily GLP-1 and GIP (glucose-dependent insulinotropic polypeptide). Under normal physiological conditions, nutrients in the gut stimulate the release of GLP-1 and GIP, leading to increased insulin secretion and decreased glucagon release from the pancreas in a glucose-dependent manner [22]. DPP-4 rapidly inactivates GLP-1 and GIP unless their degradation is prevented by Sitagliptin or other DPP-4 inhibitors, thus prolonging their effect on pancreatic islet cells. This mechanism lowers glucose levels without promoting hypoglycemia, a key safety advantage [22].
Although GLP-1 activity may modestly delay gastric emptying and increase satiety, DPP-4 inhibitors are generally weight-neutral. Sitagliptin is administered orally (100 mg once daily in patients with normal renal function) and maintains >80% DPP-4 inhibition over 24 hours [23].
1.8. Efficacy and Safety Profile
Sitagliptin and other DPP-4 inhibitors produce moderate reductions in HbA1c (typically ~0.5–1.0% decrease) when taken with metformin or other agents, making them useful second-line or third-line therapies in T2DM. They are most effective in the context of sufficient endogenous incretin production. Unlike sulfonylureas, DPP-4 inhibitors do not cause weight gain, and their glucose-dependent mechanism minimizes the risk of hypoglycemia unless combined with insulin or secretagogues [24]. Adverse effects are generally mild, including nasopharyngitis and headache. Serious adverse events such as hypersensitivity reactions and severe joint pain are rare [10]. While initial concerns were raised about pancreatitis, large trials have not confirmed a significantly increased risk. Importantly, the TECOS trial demonstrated that sitagliptin does not heighten the risk of major adverse cardiovascular events (MACE), including myocardial infarction, stroke, or cardiovascular death [9]. Although one agent in the class (saxagliptin) was associated with increased heart failure hospitalizations, this effect was not observed with sitagliptin [9].
1.9. Pharmacokinetics and Clinical Considerations
Sitagliptin is primarily excreted by the kidneys and requires dose adjustment in chronic kidney disease (CKD): 50 mg daily if the estimated glomerular filtration rate (eGFR) is <50 mL/min, and 25 mg daily for severe impairment [25]. This adjustability supports safe use in patients with moderate-to-severe CKD. Sitagliptin is also well tolerated in elderly patients and has minimal drug-drug interactions, making it suitable for polypharmacy regimens. Other DPP-4 inhibitors differ in metabolism, e.g., linagliptin is hepatically cleared and does not require renal adjustment. Sitagliptin’s ease of use (once-daily oral dosing), safety in the elderly, and neutral effects on weight and hypoglycemia risk have made it an attractive option in comprehensive T2DM management [11].
1.10. Promising Vascular Effects of Sitagliptin
Preclinical and translational studies suggest that sitagliptin exerts anti-inflammatory and vascular benefits beyond glucose lowering. In rodent models, sitagliptin reduced inflammatory responses and nociceptive behavior, confirming notable anti-inflammatory and antinociceptive effects [26]. Another study demonstrated renal protection in diabetic nephropathy by improving metabolic and renal parameters while downregulating pro-inflammatory cytokines, indicating sitagliptin may preserve kidney function through modulation of JAK-STAT signaling [27]. Evidence from diabetic rats also showed that sitagliptin alone or when combined with metformin, preserved islet architecture and improved pancreatic function, with combination therapy achieving near-normal islet morphology on histology [28].
Registry data from 2999 patients with T2DM and heart failure indicated that sitagliptin was neutral in mid-range and reduced ejection fraction subgroups, yet in HFpEF it was associated with preserved LVEF and reduced cardiovascular mortality and hospitalization [29].
2. Major Clinical Trials and Cardiovascular Outcomes
Large-scale cardiovascular outcomes trials (CVOTs) have rigorously tested sitagliptin and other DPP-4 inhibitors in patients with T2DM, as mandated by regulatory guidance to ensure new diabetes drugs are cardiovascular-protective. As shown in Table 1, these trials compare outcomes across multiple DPP-4 agents in diverse populations with T2DM and high cardiovascular risk. Below, we appraise five key trials, including those focused on sitagliptin, highlighting their design, patient demographics, primary results, and notable cardiovascular findings. The table below offers a concise comparison of these major trials, which collectively enrolled thousands of patients at elevated cardiovascular risk, forming a robust evidence base.
Table 1. Cardiovascular Outcome Trials (CVOTs) of DPP-4 Inhibitors in T2DM.
Trial |
TECOS |
SAVOR-TIMI 53 |
EXAMINE |
CARMELINA |
CAROLINA |
|---|---|---|---|---|---|
Intervention |
Sitagliptin: 100 mg/50 mg QD † |
Saxagliptin 5/2.5 mg QD † |
Alogliptin 25 mg/12.5 mg/ 6.25 mg QD † |
Linagliptin 5 mg QD |
Linagliptin 5 mg QD /Glimepiride 1–4 mg QD †† |
Target |
CV Safety |
CV Safety |
CV Safety |
CV Safety |
CV Safety |
Population |
T2DM + CVD |
T2DM + CVD |
Post-ACS |
T2DM + CKD/CVD |
T2DM + CVD risk |
Trial Status |
Completed |
Completed |
Completed |
Completed |
Completed |
Trial Design |
|||||
Number of participants |
14,671 |
16,492 |
5380 |
6979 |
6033 |
MACE Signal |
Neutral (HR 0.98) |
Neutral (HR 1.00) |
Neutral (HR 0.96) |
Neutral (HR 1.02) |
Neutral (HR 0.98) |
HF Signal |
No |
Yes (↑HF) |
Neutral |
Neutral |
Neutral |
Median follow-up |
3 years |
2.1 years |
18 months |
2.2 years |
6.3 years |
Racial/Ethnic Representation |
~3% Black, ~12% Hispanic |
3.4% Black, 21.5% Hispanic |
Underrepresented US minorities |
Race underreported |
5.4% Black, mostly Latin America |
Abbreviations: T2DM = type 2 diabetes mellitus; CVD = cardiovascular disease; CV = cardiovascular; MACE = major adverse cardiovascular events; ACS = acute coronary syndrome; CKD = chronic kidney disease; HR = hazard ratio; HF = heart failure. † Doses were adjusted based on renal function per trial protocols: Sitagliptin: 100 mg once daily, or 50 mg once daily for patients with an estimated GFR of 30–49 mL/min/1. 73 m2; Saxagliptin: 5 mg once daily, or 2.5 mg once daily for patients with an estimated GFR ≤ 50 mL/min/1.73 m2; Alogliptin: 25 mg once daily for GFR ≥ 60 mL/min/1.73 m2, 12.5 mg for GFR 30–59; 6.25 mg for GFR <30. †† Glimepiride dose (1–4 mg once daily) was individually titrated based on glycemic control in the CAROLINA trial. ↑ indicates an increased risk observed in the trial.
This table compares major cardiovascular outcome trials (CVOTs) evaluating DPP-4 inhibitors in patients with type 2 diabetes mellitus (T2DM), focusing on study duration, population characteristics, primary endpoints, cardiovascular (CV) outcomes, and heart failure (HF) signals. Results highlight the cardiovascular safety of sitagliptin and contextualize class-wide findings.
2.1. TECOS: Trial Evaluating Cardiovascular Outcomes with Sitagliptin (2015)
Design: TECOS was a double-blind, placebo-controlled CVOT designed specifically to assess sitagliptin’s cardiovascular safety in T2DM [30]. It enrolled 14,671 patients ≥ 50 years old with established CVD (e.g., coronary disease, cerebrovascular disease) and HbA1c between 6.5–8.0% on stable diabetes therapy [30]. Participants were randomized to add sitagliptin (100 mg daily, or 50 mg if moderate CKD) or placebo on top of standard care. The median follow-up was about three years. Individualized targets in both groups guided glucose management, but using other DPP-4 inhibitors or GLP-1 receptor agonists was not allowed [9]. The trial used a non-inferiority margin of 1.3 for the primary cardiovascular outcome, which is in line with FDA standards for diabetes drug safety trials [9].
Population: The average age of participants in TECOS was 65, and 29% were women. The trial enrolled patients from multiple global regions, including North America, Europe, Latin America, and Asia. About 22% of participants were Asian, but the representation of U.S. racial and ethnic minorities was low: only 3% were Black, and approximately 12% were Hispanic [9]. This underrepresentation limits generalizability to some high-risk populations. Most participants had long-standing type 2 diabetes (mean duration ~11 years) and were already on standard cardiovascular therapies, including statins, antihypertensives, and antiplatelet agents [9,30].
Outcomes: According to Table 1, TECOS demonstrated the non-inferiority of sitagliptin in major adverse cardiovascular events (MACE) compared to placebo, confirming its cardiovascular safety. The composite outcome, cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina, occurred in 11.4% of patients in the sitagliptin group and 11.6% in the placebo group over a median follow-up of three years (hazard ratio [HR] 0.98; 95% CI, 0.88–1.09; p < 0.001 for non-inferiority). Importantly, the rate of hospitalization for heart failure was identical in both groups (3.1%; HR 1.00, 95% CI, 0.83–1.20), which helped alleviate earlier concerns raised by findings from other DPP-4 inhibitor trials [9].
Sitagliptin was associated with modestly better glycemic control, averaging a 0.3% lower HbA1c to placebo, and fewer patients required escalation of glucose-lowering therapy. However, this did not translate into differences in cardiovascular outcomes, reinforcing the idea that modest improvements in glycemia do not significantly alter short-term cardiovascular risk. There were no significant differences in rates of pancreatitis or other serious adverse events. TECOS confirmed that sitagliptin did not increase the risk of cardiovascular events in patients with T2DM and established CVD, supporting its classification as a cardiovascular-neutral therapy with good tolerability and no impact on weight [9].
2.2. SAVOR-TIMI 53: Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (2013)
Design: SAVOR-TIMI 53 was a landmark CVOT evaluating saxagliptin (another DPP-4 inhibitor) vs. placebo in 16,492 patients with T2DM and either established CVD or multiple risk factors [31]. Patients were followed for a median of ~2.1 years. The primary endpoint was slightly narrower than TECOS: a 3-point MACE of cardiovascular death, nonfatal MI, or nonfatal ischemic stroke. Saxagliptin’s trial occurred earlier and set the stage for others; its results in 2013 were among the first for the DPP-4 class. This was also a non-inferiority trial (margin 1.3 HR).
Population: The cohort’s mean age was 65.1, with ~33% female. About 78% had established CVD, and the rest high risk factors. This trial included a broad international sample (sites in 26 countries). Representation of Asian patients was moderate (~12%), and Black patients 3.4% (the latter again reflecting under-enrollment relative to disease burden) [6]. Baseline HbA1c ~8.0%. Use of other cardioprotective therapies was high [31].
Outcomes: Saxagliptin, like sitagliptin, achieved non-inferiority for the primary MACE outcome. The incidence of cardiovascular (CV) death, MI, or stroke was virtually identical: 7.3% in the saxagliptin arm vs. 7.2% in placebo over 2 years (HR 1.00, 95% CI 0.89–1.12). However, a striking finding was a statistically significant increase in heart failure (HF) hospitalizations with saxagliptin. Hospitalization for heart failure occurred in 3.5% of patients on saxagliptin versus 2.8% on placebo (HR 1.27, 95% CI 1.07–1.51, p = 0.007) [31]. This ~27% relative increase in HF risk was unexpected and prompted regulatory warnings about saxagliptin in patients at risk for heart failure. Aside from HF, saxagliptin did not affect rates of ischemic events, confirming safety in terms of atherosclerotic outcomes. Still, the HF signal raised hypotheses about possible off-target effects (e.g., DPP-4’s role in cardio-renal physiology or fluid balance).
Subsequent analyses suggested the HF risk was more pronounced in patients with prior heart failure or kidney dysfunction [31]. No significant differences in pancreatitis or pancreatic cancer emerged (though numerically small increases were not statistically significant) [32]. Overall, SAVOR-TIMI 53 established that while DPP-4 inhibition does not reduce or increase atherosclerotic event risk, saxagliptin might precipitate or worsen heart failure in susceptible. This result was a class warning signal and led clinicians to use caution with saxagliptin, especially in those with a history of HF. Later trials (TECOS, CARMELINA) did not replicate this HF finding with other DPP-4 inhibitors [33,34], suggesting the effect may not be a class-wide phenomenon or is modest.
2.3. EXAMINE: Examination of Cardiovascular Outcomes with Alogliptin (2013)
Design: EXAMINE tested alogliptin, a DPP-4 inhibitor, in a high-risk post-acute coronary syndrome (ACS) population. It enrolled 5,380 patients with T2DM who had suffered a recent ACS (within 15–90 days), either myocardial infarction or unstable angina requiring hospitalization [35,36]. This scenario represents patients with very high short-term CV risk. Alogliptin or placebo was added to their standard care in a double-blind fashion, with a primary endpoint of CV death, nonfatal MI, or nonfatal stroke (3-point MACE), non-inferiority margin 1.3 [35]. Median follow-up was 18 months, shorter than other trials (owing to the high-risk ACS cohort) [36].
Population: On average, patients were 61 years old, and 32% were female. All had recent coronary events; many underwent revascularization and were on intensive medical therapy (dual antiplatelet therapy, high-dose statins, etc.). This trial had more participants from North America (~16%), Central and South America (~26%), and Europe. Ethnic minority representation was limited overall: Black participants made up only 3.4% of the cohort, despite the global burden of diabetes in Black populations. Although Hispanic participants comprised 28.6% of the population, many Latin American centers categorized race differently [6]. Kidney function was moderately impaired in some participants (EXAMINE did not exclude stage 3 CKD) [37]. Baseline HbA1c ranged between 6.5% and 11% [35].
Outcomes: EXAMINE found that alogliptin was non-inferior to placebo for the primary composite outcome. The 3-point MACE occurred in 11.3% of alogliptin vs. 11.8% of placebo patients (HR 0.96, one-sided 95%, CI upper bound 1.16, p < 0.001 for non-inferiority). In other words, there was no increase in cardiovascular events with alogliptin after ACS. Glycemic control was better in the alogliptin arm (HbA1c lower by 0.36% vs. placebo), yet this did not translate to fewer CV events during follow-up, consistent with the other trials [35]. Importantly, hospitalization for heart failure rates were not significantly different between alogliptin and placebo in EXAMINE [38]. A numerical imbalance was observed (alogliptin: 3.9% vs. placebo: 3.3%), but it did not reach statistical significance, and careful analysis suggested no clear HF hazard (unlike saxagliptin). No other safety concerns emerged; rates of pancreatitis and liver dysfunction were similar between groups [35].
EXAMINE’s results reinforced the message that DPP-4 inhibitors do not worsen cardiovascular outcomes in the short-to-medium term, even in very high-risk post-ACS patients. The trial was underpowered to detect small differences in individual endpoints, but its reassurance was valuable given the vulnerability of the population. Alogliptin’s sponsor used these findings to satisfy safety requirements [35]. Clinically, EXAMINE indicated that it is reasonable to continue or start a DPP-4 inhibitor like alogliptin in people with diabetes after an ACS without fear of harming prognosis. However, other interventions (statins, beta-blockers, ACE inhibitors, SGLT2 inhibitors, etc.) remain the mainstay for improving cardiac outcomes [35].
2.4. CARMELINA: Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (2018)
Design: CARMELINA focused on linagliptin, targeting patients with T2DM who had both high cardiovascular risk and renal disease. This placebo-controlled trial enrolled 6979 patients across 27 countries, many with established CVD and/or chronic kidney disease (mean eGFR ~55 mL/min, and >80% with albuminuria) [39]. Importantly, ~15% had no prior CVD but were high risk due to CKD, making CARMELINA more inclusive of primary prevention than earlier trials. Participants were randomized to linagliptin 5 mg daily or placebo on top of standard care and followed for a median of 2.2 years [39]. Co-primary outcomes were: (1) 3-point MACE (CV death, nonfatal MI, nonfatal stroke) for CV safety, and (2) a composite kidney outcome (time to end-stage renal disease, death due to renal failure, or ≥40% sustained decline in GFR) [39].
Population: The mean age was 65.9, with 37.1% female. Due to the CKD enrichment, this cohort had high comorbidity: nearly all had albuminuria, and many had diabetic kidney disease stages 3–4. Roughly 57% had established CVD. Geographically, CARMELINA included patients from Europe, Asia, North America, and Latin America. It had one of the highest representations of Black patients among DPP-4 trials (~5.9% Black participants), 35.8% Hispanic and ~13% Asian, though still underrepresenting Blacks relative to U.S. diabetes demographics [6]. Linagliptin’s lack of need for renal dose adjustment made it appealing to test in CKD patients.
Outcomes: Linagliptin proved safe for cardiovascular use (non-inferior) and neutral with respect to renal progression. The primary 3-point MACE occurred in 12.4% of the linagliptin group vs. 12.1% of placebo (HR 1.02, 95% CI 0.89–1.17, p < 0.001 for non-inferiority) [39]. There was no signal of harm or benefit on CV events-linagliptin neither increased nor decreased rates of MI, stroke, or CV death relative to placebo. Likewise, the secondary kidney composite outcome was virtually the same between groups (9.4% vs. 8.8%, HR 1.04, p = 0.62), indicating no added renal benefit beyond standard care (which often included renin-angiotensin system blockers; SGLT2 inhibitors were not yet widely used in CKD at the time). HF hospitalization rates were identical in linagliptin vs. placebo, consistent with TECOS and reassuring that saxagliptin’s HF risk was not seen here [34]. In terms of safety, there were no differences in overall adverse events or hypoglycemia.
A small imbalance in acute pancreatitis (0.3% vs. 0.1%) was observed but not statistically significant. CARMELINA’s clinical message is that linagliptin can be used in patients with advanced diabetic kidney disease and prior cardiovascular events without concern for excess cardiovascular or renal risk [39]. However, unlike newer agents, SGLT2 inhibitors and GLP-1 receptor agonists, linagliptin does not confer measurable cardio-renal protective benefits. Even in severe CKD, linagliptin’s glucose-lowering efficacy is maintained, which was very well tolerated in this fragile population. This trial expanded the evidence base to sicker patients and confirmed class safety across a spectrum of kidney function.
2.5. CAROLINA: Cardiovascular Outcome Study of Linagliptin vs. Glimepiride (2019)
Design: CAROLINA was unique among CVOTs in that it used an active comparator (glimepiride, a sulfonylurea) instead of a placebo. It addressed a practical clinical question: how does linagliptin compare to a commonly used oral agent (sulfonylurea) in terms of cardiovascular outcomes and overall safety? CAROLINA randomized 6042 relatively early-stage T2DM patients (drug-naïve or on metformin monotherapy) with elevated CV risk (around 40% had established CVD, others had multiple risk factors or were age ≥ 70 with risk factors) to linagliptin 5 mg vs. glimepiride (titrated to 1–4 mg) for up to 6+ years [40]. Median follow-up was 6.3 years, making it the longest DPP-4 inhibitor trial. The primary endpoint was 3-point MACE (non-inferiority test for linagliptin vs. glimepiride) [40].
Population: Mean age was 64, with 40% women, a higher female proportion than many prior trials. Because many did not have established CVD, this was a lower-risk population on average (closer to primary prevention). As a result, event rates were lower. Racial/ethnic diversity was moderate:17.1% Hispanic, 5.4% Black, and patients from 43 countries, with Europe and Latin America comprising the largest groups. Not all Latin American participants identified as Hispanic. All participants had relatively early T2DM (median duration ~6 years, baseline HbA1c ~7.2%) [40].
Outcomes: Linagliptin was found to be non-inferior to glimepiride for cardiovascular outcomes. The incidence of CV death, MI, or stroke was 11.8% with linagliptin vs. 12.0% with glimepiride over the long follow-up (HR 0.98, 95% CI 0.84–1.14). There was no significant difference in any component of the MACE outcome, essentially, the DPP-4 inhibitor and the sulfonylurea had equivalent CV profiles. This was important because sulfonylureas have been in use for decades, and their CV safety had been called into question by some observational data; CAROLINA provided reassurance that glimepiride was not worse than a neutral agent in terms of CV risk, and vice versa [40].
A key secondary finding was that linagliptin had a dramatically lower risk of hypoglycemia compared to glimepiride. The incidence of severe hypoglycemia in the linagliptin group was 0.3% compared to ~2.2% in the sulfonylurea group (>75% relative risk reduction) [41]. Non-severe hypoglycemia was also far more frequent with glimepiride (as expected with an insulin secretagogue). Weight outcomes also differed; linagliptin patients had no weight change, whereas those on glimepiride gained on average ~1.5–2 kg [42]. These results point out the trade-offs in diabetes management. While both drugs achieved similar glycemic control and CV outcomes, DPP-4 inhibition did so with fewer side effects (no hypoglycemia, and no weight gain) [43].
Rates of heart failure hospitalization were low and not significantly different between groups, although a non-significant numerical increase was observed with linagliptin compared to glimepiride (HR 1.21; 95% CI, 0.92–1.59 [40]. CAROLINA’s implications for practice were that in patients where a sulfonylurea might be considered for cost or efficacy reasons, linagliptin is an effective alternative that improves hypoglycemia control and does not contribute to CV risk [41]. This evidence supports current guidelines that favor newer agents with low hypoglycemia risk for most patients, especially the elderly. It also provided the longest-term safety data for DPP-4 inhibitors, confirming no late-emerging risks [4].
2.6. Summary of Trials
Across these trials (and reinforced by meta-analyses), the DPP-4 inhibitor class has shown a remarkably consistent neutral effect on cardiovascular outcomes. None of the trials demonstrated a reduction in major CV events or mortality with DPP-4 therapy. Glycemic improvement alone (as achieved by DPP-4 inhibitors) has not translated into macrovascular benefit over 2–6 years of follow-up. On the other hand, except for saxagliptin’s heart failure finding, no significant harm has been observed, establishing cardiovascular safety. Table 1 reinforces these findings, showing a consistent cardiovascular safety profile across major DPP-4 inhibitor trials. Meta-analysis [44] of CVOTs confirmed no increase in the composite risk of CV death, MI, or stroke with DPP-4 inhibitors, and a small increase in HF hospitalization driven by saxagliptin’s data [10]. Of note, patient demographics in these trials skewed towards older, predominantly White or Asian men with long-standing diabetes. Women typically comprised only ~25–35% of participants, and Black and Hispanic patients were underrepresented (with Black patients often < 5% of the cohorts) [6]. This gap in representation is important when generalizing results, as discussed in the next section. Nonetheless, these trials collectively give clinicians confidence that using sitagliptin or other DPP-4 inhibitors to treat hyperglycemia will neither cause nor prevent cardiovascular events on average, so other cardioprotective strategies must be employed in tandem. Meanwhile, the trials highlighted the clinical advantages of DPP-4 inhibitors (fewer hypoglycemic episodes and no weight gain vs. older drugs) pertinent to patient safety and quality of life.
2.7. Disparities in Clinical Trials and Medication Access Representation Gaps in Diabetes Clinical Trials
Despite the high burden of diabetes and cardiovascular disease (CVD) in racial/ethnic minorities, these groups have historically been underrepresented in major cardiovascular outcome trials (CVOTs) of novel diabetes therapies. The DPP-4 inhibitor trials exemplify this disparity. TECOS enrolled only ~3% Black participants and ~12% Hispanic/Latino participants, while CARMELINA, the most inclusive, reported 5.9% Black and 35.8% Hispanic enrollment. SAVOR-TIMI 53 and EXAMINE fell between these ranges, with Hispanic participation at 21.5% and 28.6%, respectively. Asian representation was relatively stronger in TECOS (~22%), but “Asian” is reported as a broad category, without separate reporting of high-risk subgroups such as South Asians [6,9]. Across studies, women comprised only 29–40% of participants, despite making up ~51% of the U.S. population during the study years.
When compared directly with U.S. Census data, these gaps become more striking. In 2018, Black Americans made up 13.4% of the U.S. population, more than double their representation in CARMELINA (5.9%) and nearly five times higher than in TECOS (~3%). By contrast, Hispanic enrollment in CARMELINA (35.8%) exceeded the 18.2% U.S. population proportion, though much of this recruitment occurred in Latin America rather than among U.S. Hispanic populations, limiting generalizability. Even in CAROLINA, which had the highest female representation (40%), women remained underrepresented by ~11% relative to the national population. Overall, participants were predominantly older adults (mean age 61–66 years), with few data available for younger patients who were disproportionately affected by barriers to care. Regional enrollment patterns also skewed heavily toward Europe and Latin America in trials such as CAROLINA, while underserved U.S. populations with high diabetes burden were underrepresented.
These disparities are not simply recruitment challenges but reflect structural racism and social determinants of health (SDOH), including limited access to specialty centers, socio-economic barriers, transportation constraints, and historical mistrust of research [6,45]. As a result, trial evidence may not fully capture the treatment responses or lived experiences of the populations most affected by T2DM and CVD. Psychosocial stressors further compound these inequities: among African Americans with type 2 diabetes, coping through internalization or reactive behaviors was linked to higher HbA1c and mental distress, whereas advocacy-based coping correlated with better self-management and resilience [46].
Addressing these gaps requires intentional, equity-focused trial design. Strategies such as community-engaged site selection, culturally competent staff training, and reducing logistical barriers can improve representation. From a health equity standpoint, ensuring diversity in research is critical so that therapeutic advances benefit all patients [45]
In the context of sitagliptin, while there is no evidence that its efficacy or safety differs by race/ethnicity, the trials were not powered to detect small subgroup effects. One positive note is that cardiovascular outcome trials showed no evidence of unique safety risks among minority patients. However, since these trials were not powered to evaluate efficacy in underrepresented groups, especially Black and Hispanic populations, future efforts must focus on improving racial/ethnic representation. Approaches such as establishing trial sites in underserved areas, training culturally competent staff, and reducing participation barriers may help bridge this evidence gap [6,47].
2.8. Sitagliptin in Practice: Access, Safety, and Use in Contemporary Diabetes Care
Even when trials show a medication like sitagliptin to be effective and safe, real-world access to that therapy can be uneven. Newer diabetes drugs are often expensive, which disproportionately impacts uninsured or under-insured patients. Incretin-based therapies (DPP-4 inhibitors, GLP-1 agonists) and SGLT2 inhibitors remain patentable and have high retail prices. Without adequate insurance coverage, many people with T2DM cannot afford these medications. This leads to treatment disparities: studies have found that Black and Latino patients, and those with lower incomes, are significantly less likely to be started on newer agents than White or higher-income patients [13]. A Commonwealth Fund study revealed that between the periods of 2003–2006 and 2017–2019, the use of DPP-4/GLP-1/SGLT2 drugs rose dramatically (from 2% to 22% of U.S. diabetes prescriptions). Still, Black adults were ~27% less likely than White adults to be on one of these new classes, even after accounting for income and insurance [13].
Disparities by insurance type are striking as well; patients with private insurance are much more likely to receive novel diabetes medications than those on Medicaid or lacking insurance. This is partly due to formulary restrictions, low reimbursement associated with Medicaid, and higher co-pays in public insurance or uninsured settings. Medicaid programs in some states have strict prior authorization criteria for costly brand-name diabetes drugs, often requiring failure on older agents first [48]. Consequently, many low-income patients remain on sulfonylureas or older insulin regimens with higher risk profiles, simply because the newer drugs are financially out of reach. These inequities have clinical consequences, patients who cannot access optimal therapies may have higher rates of hypoglycemia, weight gain, and suboptimal glucose control, which in turn contribute to worse cardiovascular outcomes [13].
Medication affordability is therefore a public health priority in diabetes care. One safety-net mechanism in the U.S. is the 340B Drug Pricing Program, which allows federally qualified health centers (FQHCs), rural health clinics, and disproportionate-share hospitals to purchase outpatient medications at steep discounts [7]. Programs like 340B have enabled many community clinics to provide expensive diabetes medications (including insulin and GLP-1 agonists) either free or at low cost to uninsured and underinsured patients. A qualitative study of patients obtaining GLP-1 injections through an FQHC 340B program showed that this access was life-changing, though not all eligible patients are reached. Unfortunately, drug manufacturer restrictions and the administrative complexity of 340B can limit its impact. Apart from 340B, patient assistance programs from pharmaceutical companies and nonprofit initiatives play a role, but these often require cumbersome applications and are not a long-term solution [49].
Insurance expansion has proven to improve access: after Medicaid expansion under the Affordable Care Act in 2014, fills of diabetes prescriptions (especially for newer agents) increased by ~39% in expansion states relative to non-expansion states. In other words, Medicaid expansion was associated with significantly greater use of DPP-4 inhibitors and other modern diabetes drugs, helping to close treatment gaps. This illustrates how policy changes can rapidly translate to more equitable care. Conversely, states that did not expand Medicaid saw stagnation in uptake of newer therapies among low-income adults [50].
Geography and healthcare infrastructure also influence access. Rural areas and inner-city areas with fewer specialists might have less frequent adoption of newer diabetes medications, particularly if primary care clinicians are less comfortable with them or if pharmacies do not stock them [51]. Lack of transportation or long travel distances can impede patients from obtaining injectable medications that require more frequent follow-up (though sitagliptin, being oral, is easier to dispense). Language barriers and health literacy also come into play. Patients who do not receive culturally and linguistically appropriate counseling may be less likely to know about or adhere to advanced treatments [52].
In summary, while sitagliptin and its class are effective tools, the benefits will not be fully realized unless underserved populations have improved access. Otherwise, we risk widening health disparities, where advantaged groups reap the benefits of medical innovation and disadvantaged groups are left behind on older, less effective, or riskier therapies. Achieving equity will require concerted efforts in health policy, healthcare delivery, and community engagement, discussed further below.
3. Clinical Considerations: Complex Patients and Sitagliptin
Managing T2DM in clinical practice, especially in patients with multiple comorbidities, requires balancing glycemic efficacy, safety, and practicality. Sitagliptin offers certain advantages in the context of polypharmacy and multimorbidity.
Polypharmacy and Hypoglycemia Risk: Patients with T2DM often take numerous medications for co-existing conditions like hypertension, dyslipidemia, coronary disease, and kidney disease. Adding diabetes medications should ideally not add significantly to pill burden or cause drug-drug interactions. Sitagliptin is a once-daily pill that is generally well-tolerated and has minimal interactions, making it easy to incorporate into complex regimens [23]. In older patients on many medications, avoiding hypoglycemia is paramount; hypoglycemic episodes can precipitate falls, cognitive impairment, or cardiac arrhythmias. Agents like sulfonylureas and insulin carry substantial hypoglycemia risk, whereas sitagliptin’s glucose-dependent action results in a very low hypoglycemia risk [53].
The American Geriatrics Society Beers Criteria recommend against certain sulfonylureas in older people due to hypoglycemia risk. DPP-4 inhibitors, by contrast, are considered safe for older adults. Studies indicate they significantly reduce hypoglycemic events compared to sulfonylureas while achieving similar glycemic goals. Thus, for a 78-year-old patient on 10 medications, substituting sitagliptin for a sulfonylurea could simplify management and improve safety (no need for intensive glucose monitoring or extra snacks to avert hypoglycemia). Polypharmacy also raises adherence issues; sitagliptin’s once-daily dosing and mild side effect profile help with adherence compared to drugs that cause GI upset or require multiple daily doses. Even though DPP-4 inhibitors can cause rare joint pains or pancreatitis, these side effects are rare and usually do not complicate general management [10].
Renal Function and Dose Adjustment: Many patients with long-standing T2DM develop chronic kidney disease, which complicates medication choices [54]. Metformin, for instance, must be dose-reduced or stopped in advanced CKD due to lactic acidosis risk, and some SGLT2 inhibitors are less effective or not indicated below certain eGFR thresholds [54]. As shown in Table 2, sitagliptin can be safely used in CKD patients with appropriate dose adjustment; the dose is reduced to 50 mg daily if GFR 30–50 mL/min, and 25 mg daily if GFR < 30 mL/min. It has been used in dialysis patients (although official labeling advises caution in ESRD). Table 2 summarizes recommended dosing adjustments based on CKD stage and eGFR, as outlined in current guidelines and prescribing information.
Table 2. Recommended Sitagliptin Dosage Adjustments by CKD Stage.
eGFR Range |
Recommended Dose |
|---|---|
≥50 mL/min |
100 mg daily |
30–49 mL/min |
50 mg daily |
<30 mL/min or ESRD |
25 mg daily |
Abbreviations: CKD = chronic kidney disease; ESRD = end-stage renal disease; eGFR = estimated glomerular filtration rate.
This table outlines recommended sitagliptin dosing based on estimated glomerular filtration rate (eGFR) thresholds, in accordance with prescribing guidelines. Dose adjustments are essential to ensure safety in patients with chronic kidney disease (CKD) or end-stage renal disease (ESRD).
CARMELINA showed linagliptin had no adverse renal effects in advanced CKD, and by class extension, sitagliptin is also considered renal-neutral [39]. There is no evidence that sitagliptin slows renal disease progression, unlike SGLT2 inhibitors or Finerenone, which do [55]. But crucially, it does not worsen kidney outcomes. In the management of a patient with Stage 3 CKD, sitagliptin is a viable option to control glucose where other drugs might be contraindicated, with dosing guided by Table 2. It also does not cause fluid retention, a problem with thiazolidinediones that can exacerbate heart failure or CKD edema. When initiating sitagliptin in CKD patients, the dose should be adjusted and renal function monitored periodically. Still, no special safety labs are required (as opposed to metformin, where one monitors eGFR for threshold cutoffs) [54].
Multimorbidity and Clinical Decision-Making: Patients with T2DM often present with co-existing cardiovascular disease, obesity, heart failure, and other comorbidities. Modern diabetes guidelines (e.g., ADA and EASD) promote a patient-centered approach. For those with established CVD or high risk, GLP-1 receptor agonists or SGLT2 inhibitors with proven cardiovascular benefit are preferred as add-ons to metformin [56]. However, not all patients can tolerate or afford these agents. GLP-1 RAs are injectables, costly, and often cause gastrointestinal side effects [57], while SGLT2 inhibitors may be contraindicated in patients with recurrent mycotic infections or eGFR <30 mL/min [58,59]. In such cases, DPP-4 inhibitors like sitagliptin offer a safer, well-tolerated alternative that modestly improves glucose without added CV or renal risk. A complex patient with ischemic cardiomyopathy, Stage 4 CKD, and T2DM may be better suited for sitagliptin, which remains effective at low GFR, is safe in HF and CKD (with dose adjustment), and avoids weight gain, unlike insulin or sulfonylureas [10]. When weight loss is a priority, GLP-1 RAs or lifestyle changes are ideal, but sitagliptin remains a reasonable fallback when those are not feasible. This comparative positioning is outlined in Table 3, which contrasts DPP-4 inhibitors with SGLT2 inhibitors and GLP-1 receptor agonists across efficacy, safety, cardiovascular, and practical domains.
Drug Affordability and Regimen Simplification: Many multimorbid patients are on complex insulin regimens. Adding a DPP-4 inhibitor like sitagliptin can reduce insulin needs and lower hypoglycemia risk. Its once-daily, side-effect–sparing profile improves adherence, especially in older adults or those with cognitive impairment. With patent expiry expected by 2026–2027, sitagliptin may soon become a more affordable and practical option in primary care.
Table 3. Comparative Profile of Glucose-Lowering Therapies.
Drug/Class |
(DPP4i) Sitagliptin |
(DPP4i) Linagliptin |
(DPP4i) Saxagliptin |
(DPP4i) Alogliptin |
SGLT2 Inhibitors (Empagliflozin, Dapagliflozin, Canagliflozin) |
GLP-1 Receptor Agonists (Semaglutide, Liraglutide, Exenatide) |
|---|---|---|---|---|---|---|
HbA1c |
~0.5–1.0% |
~0.5–1.0% |
~0.5–1.0% |
~0.5–1.0% |
~0.5–0.8% |
~1.0–1.5% |
Weight |
Neutral |
Neutral |
Neutral |
Neutral |
Modest weight loss |
Substantial weight loss (greater at obesity-dose; ~2–5 kg at diabetes-dose) |
Hypoglycemia |
Very low risk (unless combined with insulin or sulfonylurea) |
Very low risk (unless combined with insulin or sulfonylurea) |
Very low risk (unless combined with insulin or sulfonylurea) |
Very low risk (unless combined with insulin or sulfonylurea) |
Very low risk (unless combined with insulin or sulfonylurea) |
Very low risk (unless combined with insulin or sulfonylurea) |
CV (MACE) |
Neutral (TECOS) |
Neutral (CARMELINA/CAROLINA) |
Neutral (SAVOR-TIMI), ↑ HHF signal |
Neutral (EXAMINE); FDA warning for HF risk |
↓MACE (empagliflozin, canagliflozin); dapagliflozin neutral but ↓HHF |
↓ MACE |
Heart Failure |
No increase (TECOS) |
No increase (CARMELINA/CAROLINA) |
↑ HHF hospitalization |
No overall increase; class HF warning issued |
Large ↓HHF; proven in HFrEF and HFpEF (independent of diabetes) |
Largely neutral on HF hospitalization |
Use in renal impairment |
Dose adjusted by eGFR |
No renal dose adjustment |
Dose adjusted in CKD |
Dose adjusted in CKD |
Dose adjusted by eGFR |
Generally safe; no dose adjustment (exenatide is an exception) |
Dosing/route |
Oral, once daily |
Oral, once daily |
Oral, once daily |
Oral, once daily |
Oral, once daily |
Mostly injectable (weekly/daily); oral semaglutide available |
Cost/access in the US |
Brand (anticipated generic); coverage varies; prior auth often needed |
Branded; often covered |
Generic available |
Generic; low cost |
Brand only; high cost; formulary coverage improving |
High cost; prior auth common |
Use Guidance |
Preferred in older/frail patients needing low hypoglycemia risk and simplicity; adjust dose in CKD. Use with caution if there is a history of pancreatitis. |
Preferred in CKD (no dose adjustment). |
Consider only if others are not suitable; avoid if HF risk. |
Low-cost option; avoid if HF risk. |
Preferred in HF, CKD, ASCVD (agent-specific); avoid/reconsider with recurrent GU infections, volume depletion, peri-op DKA risk. |
Preferred for ASCVD/obesity/weight loss; avoid in severe GI disease, history of medullary thyroid carcinoma or MEN2. |
Abbreviations: HbA1c = glycated hemoglobin A1c; CV = cardiovascular; MACE = major adverse cardiovascular events; HF = heart failure; HHF = hospitalization for heart failure; HFrEF = heart failure with reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; ASCVD = atherosclerotic cardiovascular disease; GI = gastrointestinal; FDA = U.S. Food and Drug Administration; MEN2 = multiple endocrine neoplasia type 2. ↑ indicates an increased risk observed in the trial; ↓ indicates a reduced risk observed in the trial.
4. Public Health and Policy Implications: Improving Equitable Access
A series of public health, health system, and policy measures should be pursued to translate the benefits of therapies like sitagliptin into population health gains. Below are key recommendations to improve access and outcomes, with a focus on underserved communities and health equity:
- -
Strengthen Insurance Coverage for Diabetes Care: Ensuring patients have health insurance (Medicaid, Medicare, or private) is fundamental. States that expanded Medicaid saw significant increases in use of newer antihyperglycemic agents, including a 39% jump in DPP-4/GLP-1/SGLT2 prescriptions among low-income adults [50]. Policymakers should continue to expand Medicaid eligibility and reduce administrative barriers so that more people can obtain modern diabetes medications. Additionally, Medicare and commercial plans should be encouraged or required to include DPP-4 inhibitors and other proven therapies on formularies with affordable co-pays. Value-based insurance design, for example, $0 co-pay for medications that prevent complications could be implemented for diabetes drugs to improve adherence and long-term cost savings.
- -
Leverage 340B and Safety-Net Resources: Protecting and optimizing the 340B Drug Pricing Program is critical for ensuring affordable diabetes medications at federally qualified health centers (FQHCs) and public hospitals [60]. Clinics should expand the use of patient assistance programs and pharmacy assistance staff to help uninsured patients access sitagliptin and similar agents. Community health centers may also offer sliding-scale pricing models to improve affordability. Recent pharmaceutical industry efforts to restrict 340B contract pharmacies threaten this safety-net model and may severely limit the reach of discounted medications. Preserving the integrity of 340B and ensuring its accessibility across pharmacy networks is essential for equity, especially as sitagliptin becomes generically available. Without these protections, access to medications can remain easy for the insured, while remaining out of reach for the working poor. As reflected in Table 3, cost and access barriers are particularly stark for GLP-1 RAs and SGLT2 inhibitors, compared to the relative affordability and safety of sitagliptin.
- -
Expand Culturally Competent Diabetes Management: Investing in culturally tailored diabetes self-management and support programs can significantly improve health outcomes in high-risk communities. Programs that employ community health workers or educators who speak the language and understand the culture of their patient populations are more likely to succeed [61]. These professionals can help patients navigate complex insurance or pharmacy systems, dispel myths around medications like sitagliptin, and reinforce consistent medication adherence. At a broader level, partnerships with community organizations such as churches, barber shops, and civic groups can increase trust and awareness of available services [62]. Evidence shows that culturally competent interventions can improve diabetes control, enhance treatment uptake, and reduce healthcare disparities [63]. These approaches are especially important given the historical underrepresentation of minority groups in clinical trials and the skepticism that can result.
- -
Promote Inclusive Research and Guideline Development: Underrepresentation of racial and ethnic minorities in cardiovascular and diabetes trials continues to limit the generalizability of findings [6]. Future studies should prioritize diversity in enrollment by partnering with minority-serving institutions, utilizing mobile research clinics, and involving trusted community leaders in trial outreach [64]. Regulatory agencies and funders should mandate and incentivize diversity reporting as part of trial design and post-approval surveillance [6]. In parallel, clinical guidelines from diabetes and cardiology societies must incorporate health equity language, emphasizing cost-conscious prescribing and alternative regimens where newer agents are inaccessible. For example, in populations with unaffordable GLP-1 RAs or SGLT2is, guidelines should acknowledge DPP-4 inhibitors like sitagliptin as viable alternatives to improve glycemic control safely. Bridging the gap between evidence-based recommendations and real-world access remains a critical step toward equity.
- -
Affordability Initiatives and Drug Pricing Reform: High drug prices are at the core of access issues. Policymakers should pursue reforms such as allowing Medicare to negotiate drug prices, capping out-of-pocket costs for insulin and other diabetes medications (some of which have begun with recent legislation for insulin), and speeding the availability of safe generics. As sitagliptin faces patent expiry in the coming years, ensuring a robust generic market will drive costs down. International reference pricing or value-based pricing models could also be considered for expensive diabetes drugs that provide proven benefits (e.g., pricing drugs in proportion to the health benefit they produce). Transparent communication between pharmacists, clinicians, and patients is crucial to ensuring that cost-effective options are offered.
- -
Strengthen Integration of Diabetes and Cardiovascular Care: Given the overlapping risks of T2DM and cardiovascular disease, integrated care models are especially important. Medical homes and accountable care organizations can bring together multidisciplinary teams, including primary care, endocrinology, cardiology, pharmacy, and nutrition, to ensure patients receive comprehensive, evidence-based care. Pharmacists can play a valuable role in medication therapy management: identifying treatment gaps, simplifying regimens, and optimizing the use of agents like sitagliptin. Integrated care should also include linkages to community services that make lifestyle modifications feasible, such as exercise programs, food support, and smoking cessation resources. From a public health standpoint, supportive environments, walkable neighborhoods, access to healthy food, and culturally tailored programs are necessary to sustain health improvements beyond the clinic.
5. Discussion
5.1. Contribution of This Review
At first glance, one might question our decision to focus on sitagliptin when newer agents like GLP-1 receptor agonists and SGLT2 inhibitors have demonstrated superior cardiovascular and renal outcomes.
To compare the clinical effects of sitagliptin with other DPP-4 inhibitors and glucose-lowering agents, we created Table 3. The side-by-side comparison of sitagliptin with linagliptin, saxagliptin, and alogliptin reveals comparable HbA1c reduction across all DPP-4 agents. Unlike saxagliptin and alogliptin, sitagliptin and linagliptin have demonstrated no increased risk of heart failure hospitalization (Table 3). In addition, linagliptin is the only DPP-4 inhibitor that does not require dose adjustment in patients with CKD, as it is not eliminated by the kidneys [39,65]. Beyond clinical effects, cost considerations are highly relevant. In 2025, the Inflation Reduction Act (IRA) designated sitagliptin (Januvia) among the first 10 drugs subject to Medicare price negotiations, with projected reductions of ~70% from 2023 list prices by 2026 [66]. These negotiated prices are expected to yield substantial Medicare savings and may improve affordability for Medicare beneficiaries. In 2023, the FDA also approved Zituvio, a sitagliptin free-base formulation marketed as a lower-cost alternative to Januvia. While Zituvio has expanded access for some uninsured patients through fixed cash-pay programs, it is not considered a generic equivalent of Januvia, which remains protected under a U.S. patent until 2026. Thus, while sitagliptin is currently priced comparably to other branded DPP-4 inhibitors, its IRA designation and the eventual introduction of true generics are expected to make it the most affordable DPP-4 inhibitor in the coming years. In contrast, cardioprotective SGLT2 inhibitors and GLP-1 receptor agonists remain considerably more expensive (Table 3), with coverage varying by state and insurance plan. High out-of-pocket costs continue to disproportionately burden individuals with limited resources, contributing to adverse health outcomes among underserved populations.
This table summarizes efficacy, cardiovascular, heart failure, renal, safety, cost, and access considerations of major glucose-lowering drug classes in type 2 diabetes mellitus (T2DM). It highlights class-wide patterns while noting agent-specific findings from cardiovascular outcome trials (CVOTs).
5.2. Sitagliptin’s Role in Modern Diabetes and Cardiovascular Care
Sitagliptin’s role in the modern management of type 2 diabetes has long been viewed through the lens of its glucose-lowering ability and cardiovascular neutrality. However, this review sought to go beyond that narrative, highlighting a richer spectrum of benefits revealed in emerging literature, particularly in the areas of vascular function, multimorbidity, and equitable care. Table 4 below summarizes these effects, integrating clinical trial findings and mechanistic studies.
As can be seen from Table 4, sitagliptin exerts its glucose-lowering effects without promoting hypoglycemia or weight gain, making it a rational choice for older adults, those with frailty, or patients on complex medication regimens. Next, we consider mechanistic and microvascular effects beyond glucose lowering.
Table 4. Summary of Sitagliptin’s Clinical Benefits, Vascular Effects and Limitations.
Domain |
Observed Effects |
Supporting Data |
|---|---|---|
Glycemic Control & Weight Effects | ||
Glycemic Control |
HbA1c reduction of ~0.5–1.0% without significant hypoglycemia |
|
Weight Impact |
Weight-neutral; does not promote gain or loss |
|
Cardiovascular Outcomes | ||
Cardiovascular Safety |
Neutral for MACE (non-inferior); no increased risk of CV death, MI, stroke, or HF |
[9] |
Heart Failure Outcomes |
May benefit HFpEF patients (lower CV death and hospitalization) |
[29] |
Vascular, Endothelial & Inflammatory Effects | ||
Microvascular Protection |
May reduce urinary albumin excretion and preserve islet cell morphology |
[28] |
Endothelial Effects |
Improves NO bioavailability; reduces oxidative stress and inflammation |
|
Anti-Inflammatory Properties |
Reduces TNF-α, IL-6, and downregulates JAK-STAT pathway |
|
Pancreatic Benefits |
Preserves islet architecture, reduces damage in STZ-diabetic rats |
[28] |
Renal Outcomes & Safety Profile | ||
Renal Considerations |
Safe in CKD with dose adjustment; does not worsen renal outcomes |
|
Side Effects |
Rare: pancreatitis, joint pain; Common: nasopharyngitis, headache |
|
Patient Use, Access & Limitations | ||
Patient Populations Benefited |
Elderly, CKD, polypharmacy, low-income patients needing oral agents |
|
Access and Equity |
Available on Medicaid formularies in some states; orally administered |
|
Limitations |
No proven macrovascular benefit; outperformed by GLP-1 RAs/SGLT2is for CVD/weight loss |
|
Abbreviations: MACE = major adverse cardiovascular events; CV = cardiovascular; MI = myocardial infarction; CKD = chronic kidney disease; HFpEF = heart failure with preserved ejection fraction; STZ = streptozotocin; NO = nitric oxide; TNF-α = tumor necrosis factor alpha; IL-6 = interleukin-6.
5.3. Inflammation, Endothelial Health, and Microvascular Effects
This review also emphasized preclinical evidence pointing to sitagliptin’s anti-inflammatory and endothelial-stabilizing effects. Preclinical studies support endothelial benefits that complement the mechanistic pathways described earlier, underscoring sitagliptin’s potential vascular activity beyond glycemic effects [19,27]. These findings suggest that sitagliptin may confer vascular benefits that are not easily captured in traditional MACE endpoints. While these effects have not yet translated into statistically significant reductions in macrovascular events in humans, they may play an important role in preserving vascular health over the long term. This is further supported by its impact on nephropathy progression and islet cell morphology in diabetic rodent models, as shown in Table 4. Such data positions sitagliptin as more than just a “placeholder” therapy; it is a drug with biological activity that could be leveraged in specific patient contexts, especially when combined with other agents or where injectable options are impractical.
5.4. Cardiovascular Safety: Strengths and Limitations
The TECOS trial confirmed sitagliptin’s cardiovascular safety, with no increase in MACE or heart failure hospitalization [9], unlike saxagliptin in SAVOR-TIMI 53. This distinction emphasizes sitagliptin’s safer profile within DPP-4 inhibitors, with subgroup data hinting at benefit in HFpEF beyond glycemic neutrality [29]. For patients who cannot tolerate GLP-1 RAs or SGLT2 inhibitors or face cost and coverage barriers, sitagliptin provides a safe alternative that avoids harm, even if it does not reduce events to the same degree as newer drugs. With safety established, the practical question is how to use sitagliptin when tolerability, cost, renal dosing, or regimen simplicity are decisive.
5.5. Clinical and Public Health Implications of Sitagliptin
We now link trial findings to decision-making in the care of multimorbid patients. Sitagliptin’s trajectory from its introduction as the first oral DPP-4 inhibitor to its evaluation in cardiovascular outcomes trials provides valuable insights into the evolving paradigm of diabetes care. Clinically, the evidence confirms that glycemic control with sitagliptin is beneficial for microvascular endpoints (e.g., reducing risk of retinopathy, nephropathy progression, as shown in prior diabetes studies) and can be achieved without incurring cardiovascular harm [9]. However, improving “numbers” like HbA1c alone, especially by modest margins, does not necessarily translate into fewer heart attacks or strokes over a few years, highlighting the now well-accepted principle that comprehensive risk factor management (blood pressure, lipids, weight, tobacco cessation) and use of cardioprotective medications are required to curb CVD in diabetes [16,67] significantly.
In that context, what is the role of sitagliptin today? For many patients, sitagliptin remains a useful option to maintain glycemic targets (to prevent symptomatic hyperglycemia and microvascular damage) when first-line or more potent therapies are inadequate or unsuitable, given disadvantages such as GI side effects, being injectable, high cost, reduced effectiveness at low GFR, or increased infection risk.
In clinical scenarios involving older patients with diabetes, heart disease, and kidney disease, providers are often navigating multiple overlapping guidelines. In these cases, simplification and safety become paramount. Sitagliptin offers oral, once-daily dosing and a strong safety profile, which can reduce the treatment burden on patients and providers alike. Its minimal side effect profile and low risk of drug interactions also support adherence, especially in polypharmacy contexts. As generic sitagliptin becomes available, its use may expand in resource-constrained settings where cost has previously been a barrier. While often overlooked in major trials, these access and usability features are highly relevant in real-world and safety-net settings. Table 4 also reflects sitagliptin’s ability to meet clinical and access-related challenges in multimorbid populations. These clinical considerations lead directly to questions of access and persistence.
This table summarizes observed effects of sitagliptin across multiple domains, including glycemic control, cardiovascular safety, anti-inflammatory and endothelial benefits, renal considerations, and access equity. Evidence is drawn from clinical trials, mechanistic studies, and guideline-based treatment considerations.
5.6. Advancing Equity and Access in Diabetes Care
Access and persistence are shaped as much by delivery systems and structural conditions as pharmacology. This review also highlighted an unfortunate paradox: even as we accumulate robust evidence and new therapies for T2DM, not everyone is benefiting equally. The widening gap in utilization of newer medications between well-served and underserved populations is a call to action for the medical community and policymakers. While sitagliptin did not reduce cardiovascular events, other drugs have, and it is concerning that those transformative therapies (GLP-1 RAs, SGLT2is) are least accessible to those who could stand to gain the most (patients in minority and low-income groups who suffer higher baseline CVD risk).
This gap arises not only from cost and coverage but also from structural racism and social determinants of health (SDOH), systemic disinvestment, unequal insurance coverage, provider bias, transportation barriers, and historical mistrust of research. Bridging these inequities will require systemic reforms, including better insurance coverage, innovative payment models, and community-level interventions [62,63]. On the flip side, sitagliptin itself may become an equity-promoting agent once generic, as its ease of use and affordability could make it a staple in managing diabetes for populations that struggle with more complex regimens.
The American Diabetes Association is increasingly vocal that glucose, blood pressure, and lipid control medications must be affordable as a matter of health equity and outcome improvement [68], with the hope that by making these therapies accessible, fewer disparities in complications like amputations, kidney failure, and cardiovascular deaths will be seen. The collective challenge is delivering the medications to the right patient at the right time.
5.7. Practical Solutions: Community and Systems Implementation
The equity gaps discussed throughout this article are produced not only by cost and coverage issues but also by structural racism and SDOH-systemic disinvestment, unequal insurance coverage, provider bias, transportation barriers, and mistrust of care and research. Closing them requires pragmatic regimens and care-delivery models that fit patients’ daily realities.
-
System-Level Actions (Payers/Health Systems): Streamline access to low-risk oral agents by removing or simplifying prior authorization; cap copays; offer 90-day fills and mail-order; leverage 340B pricing where applicable; and support medication synchronization, adherence packaging, and pharmacist-led medication therapy management [60,69,70,71]. These approaches consistently improve refill persistence and adherence for chronic medications and are feasible in safety-net settings. When injectables are impractical because of intolerance, complexity, or cost, a once-daily oral option with very low hypoglycemia risk and renal dose flexibility (e.g., sitagliptin) can help maintain glycemic targets without adding cardiovascular harm in usual care [9,30].
-
Community Implementation (Trusted Touchpoints). Partner with events or programs people already trust and attend:
- -
-
Faith-based “church health days” (African American/Hispanic communities): On-site blood pressure and blood glucose screening, diabetes education, cultural tailoring, health coaching, group sessions, inclusion of spiritual components, home monitoring, navigator enrollment in discount programs, same-day appointment scheduling, and medication refill support [72,73]
- -
-
Barbershops/beauty salons: pharmacist-barber partnerships demonstrate large BP reductions among Black men [74]; this model can be adapted with brief BP checks, QR codes for low-cost medication programs, and on-site appointment booking [62].
- -
-
Culturally anchored events (e.g., Mexican fiestas/Latino festivals): promotores de salud booths providing bilingual counseling, point-of-care glucose/A1c checks, linkage to FQHCs, community health worker support, and help initiating or continuing oral agents when injections or cost are barriers [75].
- -
-
Tribal powwows/Native community health fairs: collaborations with tribal health programs for travel vouchers, refill synchronization, and language-concordant adherence support, aligned with tribal leadership priorities.
-
Similar principles apply with regional adaptations in many low- and middle-income settings. Injectables are often scarce or unaffordable, and clinic capacity is limited. Simple, oral, once-daily regimens with very low hypoglycemia risk and renal dose flexibility are more feasible to scale. Practical levers include task-shifting to nurses/community health workers using standardized protocols; pooled procurement; and basic adherence supports (blister packs, medication synchronization, brief teach-back in local languages at clinics, hospitals, and community health fairs). Programs can also offer transport vouchers, small conditional cash transfers for on-time refills, and targeted outreach through trusted settings (e.g., churches, fiestas, markets, tribal events).
Care often breaks down for patients at predictable friction points such as pharmacy costs, transportation for refills, language mismatch, and regimen complexity. Pairing an affordable, once-daily oral therapy with patient navigators, language-concordant counseling, and easy refills (e.g., 90-day supplies, mail-order, medication synchronization) reduces these barriers. It improves persistence, especially in older, multimorbid, and safety-net populations. Equally important is engaging communities directly: conduct needs assessments and listening sessions, and co-design tailored programs with community members to identify priorities, refine what works, and adapt interventions locally.
The impact of intervention strategies can be measured across five areas: access/adherence (time-to-first fill, refill persistence); process/quality (retinal exam; annual kidney evaluation with eGFR and urine albumin-creatinine ratio; statin initiation/adherence); intermediate outcomes (HbA1c change at 6–12 months, BP control, statin use); hard outcomes & equity (rates per 10,000 adults with diabetes of DKA/HHS, MI/stroke, heart failure, ESRD starts, amputations, and all-cause/CVD mortality, each stratified by race/ethnicity, language, and insurance); and population signal (annual incidence of diagnosed diabetes in the service area). Track clinic attendance and program enrollment to show reach [76,77].
5.8. Translational Considerations
Several factors likely explain why vascular/anti-inflammatory effects in animals have not yielded MACE reductions in human trials. Animal models only partially mirror human atherosclerosis and diabetic vasculopathy (differences in plaque biology, immune milieu, vascular beds, and time course). For example, while multiple mouse models are used to study atherosclerosis, most of these models do not develop myocardial infarctions [78]. Major CVOTs such as TECOS were pragmatic “usual-care” safety studies with glycemic equipoise (small HbA1c differences), high background use of statins/ACE inhibitors/ARBs/antiplatelets, and heterogeneous adherence conditions that dilute any incremental macrovascular effect of a glucose-lowering drug that is otherwise CV-neutral [9]. Even within trials, real-world heterogeneity and adherence variability further attenuate effects observed under tightly controlled preclinical conditions. These factors explain why signals seen at the bench have not produced event reductions in hard cardiovascular events at the bedside [78].
5.9. Future Research and Knowledge Gaps
Despite sitagliptin’s safety and efficacy, major CVOTs were not powered to assess outcomes across racial and ethnic minority groups. As noted in the disparities sections and reiterated in Table 4, limited representation undermines the ability to detect subgroup-specific effects, reinforcing the need for more inclusive designs in future research.
Hypertension is a central risk factor for CVD, and its burden is unevenly distributed across racial and ethnic lines. The prevalence of hypertension among the African American population in the U.S. is well established. Black adults in the U.S. experience earlier onset, more severe hypertension, and worse control than their White counterparts, a pattern shaped not solely by biology, but by persistent social stressors. The Jackson Heart Study, the largest single-site cohort of African Americans, found that cumulative exposure to discrimination across multiple domains significantly increased the risk of developing hypertension, particularly among women [79,80]. These findings align with meta-analytic evidence linking perceived racial discrimination to elevated nighttime blood pressure, revealing how chronic psychosocial stress may contribute to both hypertension and diabetes progression [81].
Disparities extend beyond African Americans. Among Hispanic populations, Afro-Caribbean subgroups (e.g., Puerto Rican, Dominican) face significantly higher hypertension risk compared to Mexican Americans. Asian Americans are least likely to achieve blood pressure control despite high treatment rates [79]. American Indian and Alaska Native (AI/AN) populations also face profound health inequities rooted in structural racism. As highlighted in a 2020 AHA presidential advisory, AI/AN communities experience persistent disparities in life expectancy and cardiovascular outcomes inequities that are shaped by entrenched systems of discrimination, marginalization, and disinvestment [82]. These disparities have been exacerbated by exclusion from research and limited access to upstream social and clinical resources. Future research should prioritize:
- -
Subgroup analyses or new trials focused on African American and Hispanic patients.
- -
Longitudinal vascular imaging studies to validate endothelial benefits observed in animal models.
- -
Combination studies assessing sitagliptin plus SGLT2 inhibitors in patients who can’t use GLP-1 RAs.
- -
Cost-effectiveness analyses in Medicaid and FQHC populations to guide formulary decisions.
Finally, as sitagliptin approaches generic availability in the U.S. (~2026–2027), real-world uptake and impact on equity should be monitored.
5.10. Limitations
Sitagliptin, despite its strengths, has important limitations. It does not reduce major adverse cardiovascular events or slow renal decline beyond glycemic effects. It lacks weight loss benefits, which are particularly desirable in many patients with obesity-related diabetes. These limitations, summarized in the final row of Table 4, should guide appropriate selection, particularly in younger patients or those with high CVD risk, where GLP-1 RAs or SGLT2is are preferred. This review also carries inherent limitations. While it incorporates animal and human data, the vascular benefits discussed are not yet validated in large prospective trials, and extrapolating mechanistic effects to macrovascular benefit is limited by model–human differences, CVOT design features, and patient heterogeneity. The mechanistic and equity-focused perspectives are intended to enhance synthesis but are interpretive rather than meta-analytic. We also acknowledge that some of the studies cited, while promising, remain preliminary and warrant cautious translation to practice.
6. Conclusions
This review aims to address the logical and equitable place of sitagliptin in a therapeutic landscape dominated by organ-protective agents The totality of evidence shows that sitagliptin provides reliable glycemic control with cardiovascular safety, weight neutrality, and very low hypoglycemia risk; mechanistic and preclinical signals suggest vascular effects, although large trials to date demonstrate cardiovascular neutrality.
Sitagliptin remains a practical option when GLP-1 receptor agonists or SGLT2 inhibitors are contraindicated, unaffordable, or not tolerated, owing to once-daily oral dosing, renal dose flexibility, and excellent tolerability. While it does not reduce macrovascular events like some newer agents, controlling hyperglycemia helps limit microvascular injury. Table 4 serves as a roadmap to its contribution: a dependable, well-tolerated, accessible therapy that can simplify care for complex, multimorbid patients. Its cardiovascular neutrality often viewed as a limitation can be a strength where safety and tolerability are paramount. Used alongside lifestyle change and comprehensive risk-factor control, sitagliptin supports individualized care without adding treatment burden.
Importantly, this review highlights sitagliptin’s overlooked potential in advancing health equity. As generic versions become available, sitagliptin may shift from a second-line standby to a frontline option for underserved populations. Its impact will depend not only on clinical efficacy, but also on whether health systems ensure access, affordability, and inclusive research. The broader lesson is clear: advances in diabetes pharmacotherapy must be matched by advances in health delivery and policy. Only then will the full promise of agents like sitagliptin be realized improving outcomes for all, especially those historically left behind.
Author Contributions
Conceptualization, A.M.O., F.E.M. and N.S.Z.; Methodology, A.M.O., F.E.M. and N.S.Z.; Investigation, A.M.O. and N.S.Z.; Data Curation, A.M.O., F.E.M. and N.S.Z.; Writing—Original Draft Preparation, A.M.O.; Writing—Review & Editing, A.M.O., F.E.M. and N.S.Z.; Visualization, A.M.O. and N.S.Z.; Supervision, N.S.Z. All authors have read and agreed to the published version of the manuscript.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare no competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Chakraborty S, Verma A, Garg R, Singh J, Verma H. Cardiometabolic risk factors associated with type 2 diabetes mellitus: a mechanistic insight. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231220780. [Google Scholar]
- World Health Organization (WHO). Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 13 June 2025). [Google Scholar]
- Martin SS, Aday AW, Allen NB, Almarzooq ZI, Anderson CA, Arora P, et al. Heart disease and stroke statistics: A report of US and global data from the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar]
- Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar]
- CDC. National Diabetes Statistics Report. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed on 13 June 2025). [Google Scholar]
- Sinclair, MR Ardehali M, Diamantidis CJ, Corsino L. The diabetes cardiovascular outcomes trials and racial and ethnic minority enrollment: impact, barriers, and potential solutions. Front. Public Health 2024, 12, 1412874. [Google Scholar]
- Wagner AA, Whitner JB, Williams AC, Hirt KL, Miracle TL, Valentino AS. Community health center 340B program: a qualitative study of the experiences of patients with diabetes. Innov. Pharm. 2023, 14, 10-24926. [Google Scholar]
- Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 2011, 34, S276–S278. [Google Scholar]
- Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of Sitagliptin on cardiovascular outcomes in type 2 diabetes. NEJM 2015, 373, 232–242. [Google Scholar]
- Kasina SV, Baradhi KM. Dipeptidyl peptidase IV (DPP IV) inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Florentin M, Kostapanos MS, Papazafiropoulou AK. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment. World J. Diabetes 2022, 13, 85–96. [Google Scholar]
- Illinois Department of Healthcare and Family Services. Medicaid preferred drug list. Available online: https://hfs.illinois.gov/medicalproviders/pharmacy/preferred.html (accessed on 13 June 2025). [Google Scholar]
- The Commonwealth Fund. Disparities in the Use of New Diabetes Medications: Widening Treatment Inequality by Race and Insurance Coverage. Available online: https://www.commonwealthfund.org/publications/issue-briefs/2022/jun/disparities-use-new-diabetes-medications-treatment-inequality (accessed on 13 June 2025). [Google Scholar]
- Rodriguez-Araujo G, Nakagami H. Pathophysiology of cardiovascular disease in diabetes mellitus. Cardiovasc. Endocrinol. Metab. 2018, 7, 4–9. [Google Scholar]
- Larsen TS, Jansen KM. Impact of obesity-related inflammation on cardiac metabolism and function. J. Lipid Atheroscler. 2021, 10, 8–23. [Google Scholar]
- Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar]
- An Y, Xu BT, Wan SR, Ma XM, Long Y, Xu Y, et al. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar]
- Magenta A, Greco S, Capogrossi MC, Gaetano C, Martelli F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar]
- Zong Y, Wang X, Zhang Y, Tan N, Zhang Y, Li L, et al. Sitagliptin ameliorates Creb5/lncRNA ENSMUST00000213271-mediated vascular endothelial dysfunction in obese mice. Cardiovasc. Drugs Ther. 2024, 38, 679–691. [Google Scholar]
- Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ, Res. 2018, 122, 624–638. [Google Scholar]
- Golla MS, Shams P. Heart failure with preserved ejection fraction (HFpEF). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar]
- Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, et al. Effect of single oral doses of Sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J. Clin. Endocrinol. Metabol. 2006, 91, 4612–4619. [Google Scholar]
- Feingold KR. Oral and injectable (non-insulin) pharmacological agents for the treatment of type 2 diabetes. In South Dartmouth (MA): Available online: The best Clinical Endocrinology source in the world; Feingold KR, Ahmed SF, Anawalt B, et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2024. [Google Scholar]
- Eligar VS, Bain SC. A review of Sitagliptin with special emphasis on its use in moderate to severe renal impairment. Drug Des. Dev. Ther. 2013, 7, 893–903. [Google Scholar]
- Hajhashemi V, Sadeghi H, Karimi Madab F. Anti-inflammatory and antinociceptive effects of Sitagliptin in animal models and possible mechanisms involved in the antinociceptive activity. Korean J. Pain 2024, 37, 26–33. [Google Scholar]
- Al-Qabbaa SM, Qaboli SI, Alshammari TK, Alamin MA, Alrajeh HM, Almuthnabi LA, et al. Sitagliptin mitigates diabetic nephropathy in a rat model of streptozotocin-induced type 2 diabetes: possible role of PTP1B/JAK-STAT pathway. Int. J. Mol. Sci. 2023, 24, 6532. [Google Scholar]
- Shawky LM, Morsi AA, El Bana E, Hanafy SM. The biological impacts of Sitagliptin on the pancreas of a rat model of type 2 diabetes mellitus: drug interactions with Metformin. Biology 2019, 9, 6. [Google Scholar]
- Enzan N, Matsushima S, Kaku H, Tohyama T, Nagata T, Ide T, et al. Beneficial effects of dipeptidyl peptidase-4 inhibitors on heart failure with preserved ejection fraction and diabetes. JACC Asia 2023, 3, 93–104. [Google Scholar]
- American College of Cardiology. Trial Evaluating Cardiovascular Outcomes with Sitagliptin—TECOS. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2015/06/08/15/55/TECOS (accessed on 13 June 2025). [Google Scholar]
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar]
- Raz I, Bhatt DL, Hirshberg B, Mosenzon O, Scirica BM, Umez-Eronini A, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor Saxagliptin. Diabetes Care 2014, 37, 2435–2441. [Google Scholar]
- Xia C, Goud A, D’Souza J, Dahagam C, Rao X, Rajagopalan S, et al. DPP4 inhibitors and cardiovascular outcomes: safety on heart failure. Heart Fail. Rev. 2017, 22, 299–304. [Google Scholar]
- McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation 2019, 139, 351–361. [Google Scholar]
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar]
- Cavender MA, White WB, Liu Y, Massaro JM, Bergenstal RM, Mehta CR, et al. Total cardiovascular events analysis of the EXAMINE trial in patients with type 2 diabetes and recent acute coronary syndrome. Clin. Cardiol. 2018, 41, 1022–1027. [Google Scholar]
- American College of Cardiology. Examination of Cardiovascular Outcomes with Alogliptin Versus Standard of Care—EXAMINE. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2013/10/06/21/16/EXAMINE (accessed on 13 June 2025). [Google Scholar]
- Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking Alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015, 385, 2067–2076. [Google Scholar]
- Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of Linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA randomized clinical trial. JAMA 2019, 321, 69–79. [Google Scholar]
- Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of Linagliptin vs Glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA 2019, 322, 1155–1166. [Google Scholar]
- Marx N, Kolkailah AA, Rosenstock J. Hypoglycemia and cardiovascular outcomes in the CARMELINA and CAROLINA trials of linagliptin: A secondary analysis of randomized clinical trials. JAMA Cardiol. 2024, 9, 134–143. [Google Scholar]
- American Family Physician. Linagliptin and Glimepiride Equally Effective for Adults with Type 2 Diabetes Mellitus. Available online: https://www.aafp.org/pubs/afp/issues/2020/0301/p309a.html#:~:text=Linagliptin%20and%20Glimepiride%
20Equally%20Effective,stroke%20in%20adults%20with (accessed on 13 June 2025). [Google Scholar] - Rosenstock J, Kolkailah AA, McGuire DK, Espeland MA, Mattheus M, Pfarr E, et al. Incident and recurrent hypoglycaemia with Linagliptin and Glimepiride over a median of 6 years in the CAROLINA cardiovascular outcome trial. Diabetes Obes. Metab. 2023, 25, 1453–1463. [Google Scholar]
- Sinha B, Ghosal,S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res. Clin. Pract. 2019, 150, 8–16. [Google Scholar]
- Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control 2016, 23, 327–337. [Google Scholar]
- McLaurin N, Tabibi D, Wang T, Alhalimi T, Lehrer HM, Harrison L, et al. Coping with discrimination among African Americans with type 2 diabetes: factor structure and associations with diabetes control, mental distress, and psychosocial resources. PCD 2024, 21, E06. [Google Scholar]
- Cai X, Lin C, Yang W, Dagogo-Jack S, Ji L. Cardiovascular outcomes of antidiabetes medications by race/ethnicity: a systematic review and meta-analysis. J. Diabetes Complicat. 2021, 35, 107980. [Google Scholar]
- Bepo L, Nguyen OK, Makam AN. Disparities in use of novel diabetes medications by insurance: a nationally representative cohort study. J. Gen. Intern. Med. 2024, 39, 2987–299. [Google Scholar]
- American Diabetes Association. (2022). Financial Assistance Available to People Living with Diabetes Through New Co-Pay Relief Fund Supported by ADA. Available online: https://diabetes.org/newsroom/financial-assistance-available-to-people-living-with-diabetes-through-new-co-pay-relief-fund-supported-by-ADA (accessed on 13 June 2025). [Google Scholar]
- Myerson R, Lu T, Tonnu-Mihara I, Huang ES. Medicaid eligibility expansions may address gaps in access to diabetes medications. Health Aff. 2018, 37, 1200–1207. [Google Scholar]
- Foss R, Fischer K, Lampman MA, Laabs S, Halasy M, Allen SV, et al. Disparities in diabetes care: differences between rural and urban patients within a large health system. Ann. Fam. Med. 2023, 21, 234–239. [Google Scholar]
- Aubrey-Basler K, Bursey K, Pike A, Penney C, Furlong B, Howells M, et al. Interventions to improve primary healthcare in rural settings: A scoping review. PloS ONE 2024, 19, e0305516. [Google Scholar]
- Ommen ES, Xu L, O'Neill EA, Goldstein BJ, Kaufman KD, Engel SS. Comparison of treatment with Sitagliptin or sulfonylurea in patients with type 2 diabetes mellitus and mild renal impairment: a post hoc analysis of clinical trials. Diabetes Ther. 2015, 6, 29–40. [Google Scholar]
- de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, et al. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar]
- Blazek O, Bakris GL. The evolution of “pillars of therapy” to reduce heart failure risk and slow diabetic kidney disease progression. AHJ Plus Cardiol. Res. Pract. 2022, 19, 100187. [Google Scholar]
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1670. [Google Scholar]
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar]
- Adimadhyam S, Schumock GT, Calip GS, Smith Marsh DE, Layden BT, Lee TA. Increased risk of mycotic infections associated with sodium-glucose co-transporter 2 inhibitors: a prescription sequence symmetry analysis. Br. J. Clin. Pharmacol. 2019, 85, 160–168. [Google Scholar]
- Simes BC, MacGregor GG. Sodium-glucose cotransporter-2 (SGLT2) inhibitors: a clinician's guide. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2125–2136. [Google Scholar]
- Illinois Primary Healthcare Association. Understanding 340B. Available online: https://www.iphca.org/wp-content/uploads/2024/04/340B-Basics-Illinois.pdf (accessed on 13 June 2025). [Google Scholar]
- Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Curr. Diab. Rep. 2013, 13, 163–171. [Google Scholar]
- Singh H, Fulton J, Mirzazada S, Saragosa M, Uleryk EM, Nelson ML. Community-based culturally tailored education programs for black communities with cardiovascular disease, diabetes, hypertension, and stroke: systematic review findings. J. Racial Ethn. Health Disparities 2023, 10, 2986–3006. [Google Scholar]
- Dragomanovich HM, Shubrook JH. Improving cultural humility and competency in diabetes care for primary care providers. Clin. Diabetes 2021, 39, 220–224. [Google Scholar]
- Hole MK, Letchuman S, Chang A, Berry LL. Community health partners in unexpected places. MCP 2023, 98, 1833–1841. [Google Scholar]
- Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*). Diabetes Obes. Metab. 2011, 13, 939–946. [Google Scholar]
- Li P, Ali MK, Narayan KMV, Umpierrez GE, Fonseca VA, Shi L, et al. Value-Based Pricing and Its Implications for the Newly Announced Medicare Negotiated Price Under the Inflation Reduction Act. Diabetes Care 2025, 48, e44–e46. [Google Scholar]
- Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: A scientific statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar]
- American Diabetes Association. We're Putting People First. Available online: https://diabetes.org/about-us/health-access-commitment (accessed on 13 June 2025). [Google Scholar]
- Liberman JN, Girdish C. Recent trends in the dispensing of 90-day-supply prescriptions at retail pharmacies: implications for improved convenience and access. Am. Health Drug Benefits 2011, 4, 95–100. [Google Scholar]
- Lauffenburger JC, Franklin JM, Krumme AA, Shrank WH, Matlin OS, Spettell CM, et al. Predicting Adherence to Chronic Disease Medications in Patients with Long-term Initial Medication Fills Using Indicators of Clinical Events and Health Behaviors. J. Manag. Care Spec. Pharm. 2018, 24, 469–477. [Google Scholar]
- Lin Q, Mathers A, Tilli T, Baker J, Bhaidani S, Grootendorst P, et al. Implementation of the appointment-based model in community pharmacies: An analysis of refills and adherence. Res. Soc. Adm. Pharm. 2023, 19, 1286–1291. [Google Scholar]
- Rhodes EC, Chandrasekar EK, Patel SA, Narayan KV, Joshua TV, Williams LB, et al. Cost-effectiveness of a faith-based lifestyle intervention for diabetes prevention among African Americans: A within-trial analysis. Diabetes Res. Clin. Pract. 2018. 146, 85–92. [Google Scholar]
- Maroney K, Laurent J, Alvarado F, Gabor A, Bell C, Ferdinand K, et al. Systematic review and meta-analysis of church-based interventions to improve cardiovascular disease risk factors. Am. J. Med. Sci. 2023. 366, 199–208. [Google Scholar]
- Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, et al. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. N. Engl. J. Med. 2018, 378, 291–1301. [Google Scholar]
- Little TV, Wang ML, Castro EM, Jiménez J, Rosal MC. Community health worker interventions for Latinos with type 2 diabetes: a systematic review of randomized controlled trials. Curr. Diab. Rep. 2014, 14, 558. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2025. Diabetes Care. 2025, 48, 14-S26. [Google Scholar]
- Centers for Medicare & Medicaid Services (CMS), Center for Medicare. Medicare 2025 Part C & D Star Ratings Technical Notes. 2024. Available online: https://www.cms.gov/files/document/2025-star-ratings-technical-notes.pdf (accessed on 12 September 2025). [Google Scholar]
- von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, et al. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017, 25, 248–261. [Google Scholar]
- Ogunniyi MO, Commodore-Mensah Y, Ferdinand KC. Race, ethnicity, hypertension, and heart disease: JACC focus Seminar 1/9. J. Am. College Cardiol. 2021, 78, 2460–2470. [Google Scholar]
- Forde AT, Sims M, Muntner P, Lewis T, Onwuka A, Moore K, et al. Discrimination and hypertension risk among African Americans in the Jackson Heart Study. Hypertension 2020, 76, 715–723. [Google Scholar]
- Dolezsar CM, McGrath JJ, Herzig AJ, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014, 33, 20–34. [Google Scholar]
- Churchwell K, Elkind MS, Benjamin RM, Carson AP, Chang EK, Lawrence W, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation 2020, 142, e454–e468. [Google Scholar]