Found 1 results
Article
08 September 2023Synthesis and Characterization of Cyclic Carbonate End-Functional Linear and Star Polyesters via Ring-Opening Polymerization
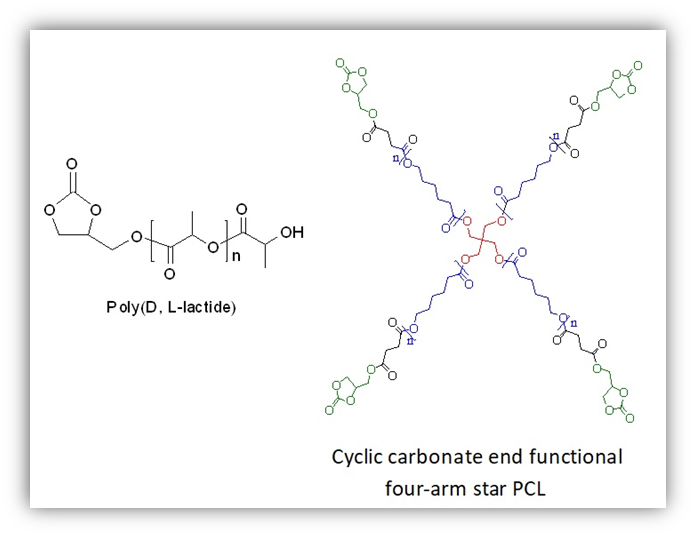
Well-defined α-(cyclic carbonate), ω-hydroxyl heterotelechelic poly (D,L-lactide)s (PDLLAs) were prepared with good end-group fidelity by ring-opening polymerization (ROP) of D,L-lactide catalyzed by organo catalyst namely, N,N′ dimethyl amino pyridine (DMAP) in conjunction with a renewable, functional bio-based initiator namely glycerol 1,2-carbonate (GC) in bulk at 135 °C with 82% yield. In the case of GC/DMAP catalyzed polymerizations, the HO-PDLLA-COOH series was not observed in MALDI TOF mass analysis unlike as obtained due to transesterification reactions when catalyzed by GC/Sn(Oct)2. Also, cyclic carbonate end-functional 4-arm star poly(ε-caprolactone) (PCL) was prepared via coupling of GC with (PCL-COOH)4 at room temperature in the presence of N,N′-dicyclohexylcarbodiimide (DCC) and DMAP. Quantitative conversion of hydroxyl functionality in (PCL-OH)4 to carboxylic acid and then to cyclic carbonate functionality was achieved with 90% yield for low molecular weight 4-arm star PCL confirmed by NMR, FT-IR, and MALDI TOF mass spectroscopy.