Found 3 results
Article
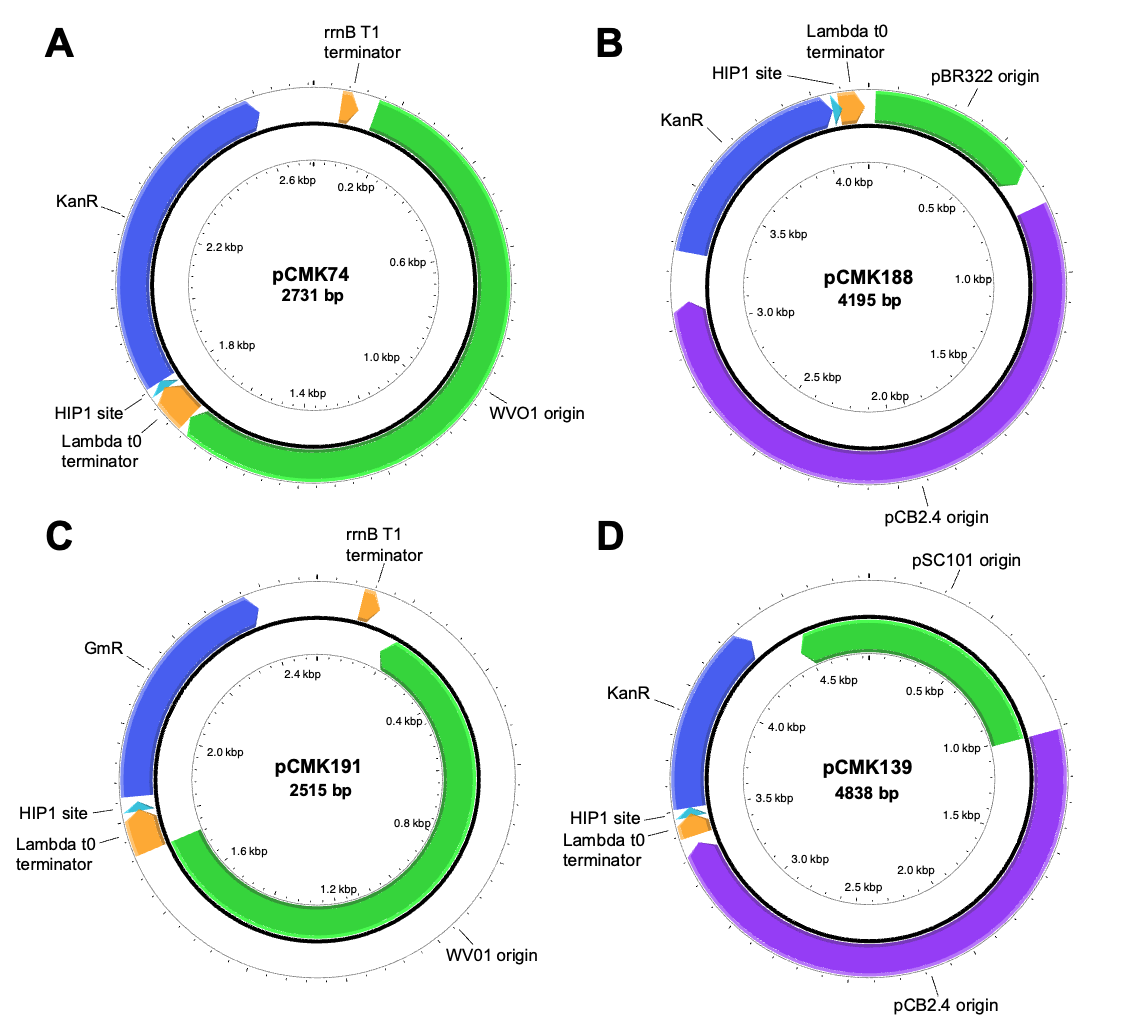
26 August 2024Delivery of Novel Replicating Vectors to Synechococcus sp. PCC 7002 Via Natural Transformation of Plasmid Multimers
In most cyanobacteria, genetic engineering efforts currently rely upon chromosomal integration; a time-consuming process due to their polyploid nature. To enhance strain construction, here we develop and characterize two novel replicating plasmids for use in Synechococcus sp. PCC 7002. Following an initial screen of plasmids comprising seven different origins of replication, two were found capable of replication: one based on the WVO1 broad host range plasmid and the other a shuttle vector derived from pCB2.4 from Synechocystis sp. PCC 6803. These were then used to construct a set of new replicating plasmids, which were shown to be both co-transformable and stably maintained in PCC 7002 at copy numbers between 7–16 and 0.6–1.4, respectively. Lastly, we demonstrate the importance of using multimeric plasmids during natural transformation of PCC 7002, with higher order multimers providing a 30-fold increase in transformation efficiency relative to monomeric plasmids. Useful considerations and methods for enhancing multimer content in plasmid samples are also presented.
Article
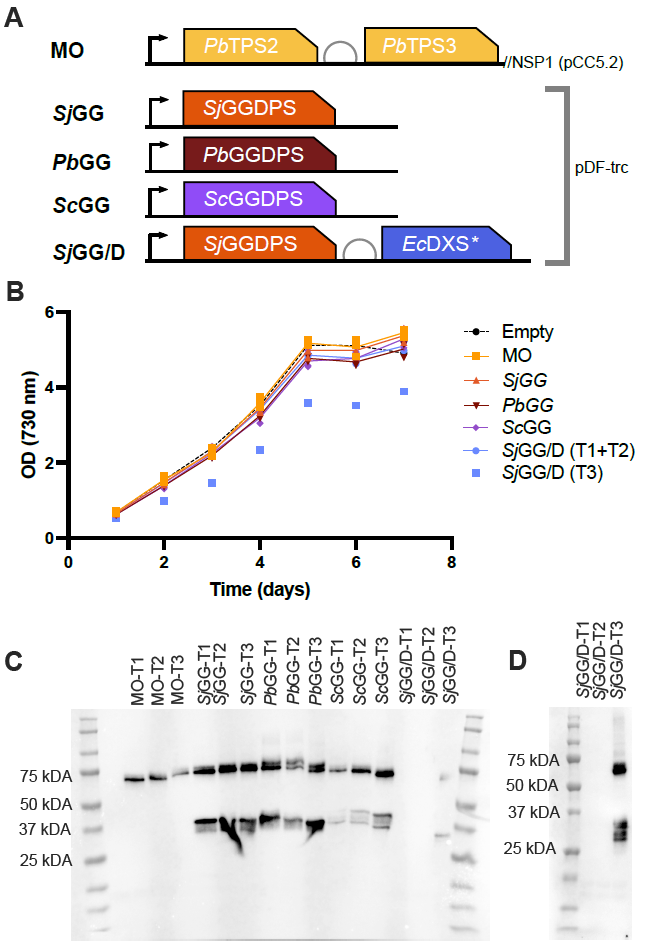
22 March 2024Modulation of the MEP Pathway for Overproduction of 13-R-manoyl Oxide in Cyanobacteria
The cyanobacterium Synechocystis sp. PCC 6803 has gained scientific interest for its potential to use solar energy and atmospheric CO2 for the production of high-value chemicals like pharmaceuticals, flavors, and fragrances. Forskolin is a diterpenoid found in the root cork of the plant Plectranthus barbatus and its biosynthetic pathway is initiated by two terpene synthases that convert geranylgeranyl diphosphate (GGDP) into the precursor 13-R-manoyl oxide (13-R-MO). Using the cyanobacterium Synechocystis sp. PCC 6803 as host, we expressed the two terpene synthases resulting in the synthesis of 0.83 mg/L 13-R-MO. Three different geranylgeranyl diphosphate synthases (GGDPSs) were selected for screening; a prokaryotic (Synechococcus sp. JA-3-3Ab (Sj)), a yeast (Saccharomyces cerevisiae (Sc)), and a plant (P. barbatus (Pb)) derived GGDPS. Strains containing the prokaryotic Sj- or the yeast ScGGDPS consistently yielded more 13-R-MO than the base strain. By overexpression of 1-Deoxy-D-xylulose-5-phosphate synthase (DXS) positioned at the entry of the 2-C-methyl-d-erythritol 4-phosphate pathway (MEP) together with the prokaryotic SjGGDPS, the 13-R-MO titer was increased 11-fold to reach 9.7 mg/L by boosting the synthesis of GGDP, the direct substrate for the diterpenoid synthases. We further show that application of a n-dodecane overlay to remove 13-R-MO from the culture medium provided a 2–3 fold increase of the 13-R-MO in a separate cultivation system.
Review
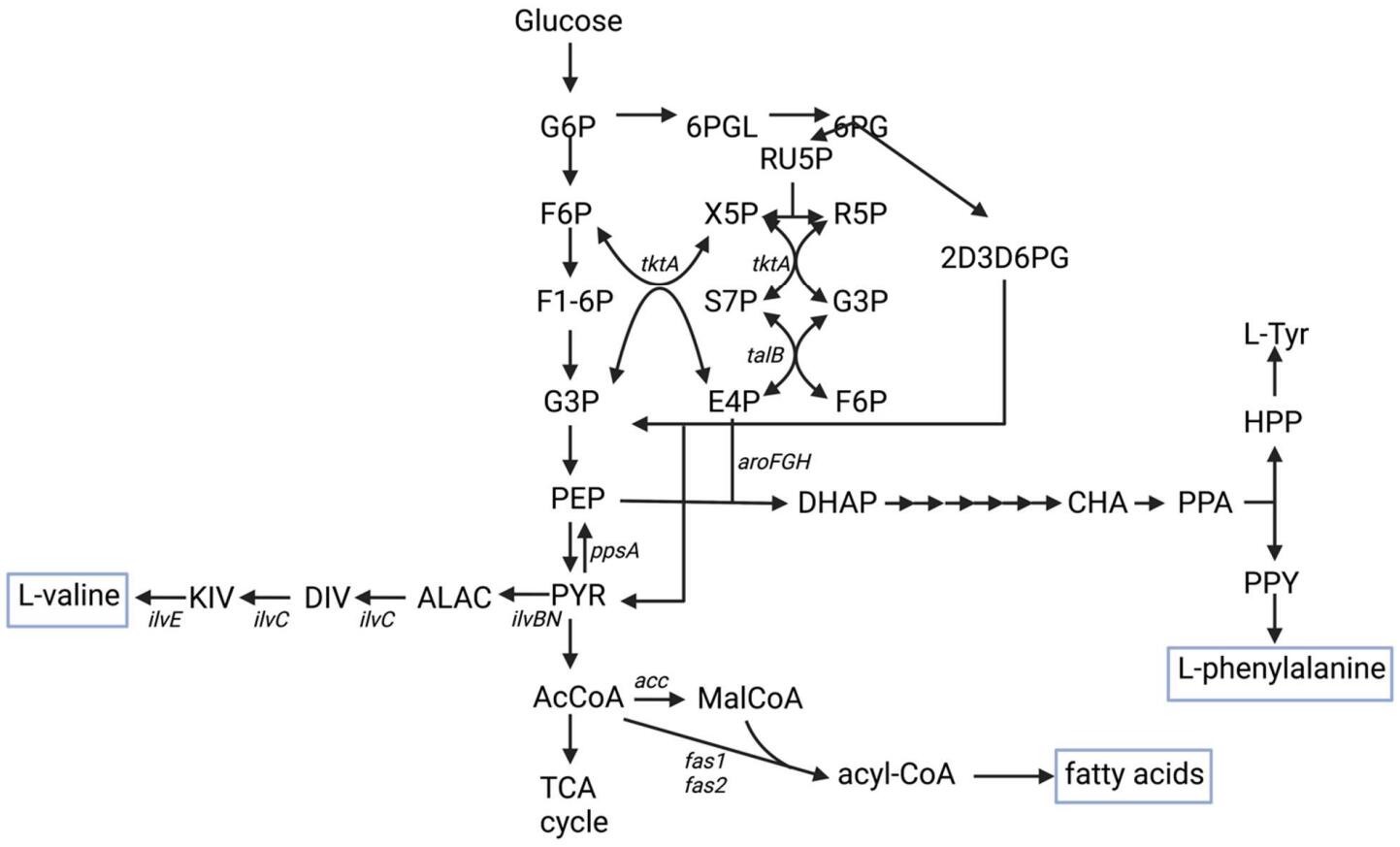
16 February 2023Increasing Nutritional Value of Cyanobacteria by Engineering Valine, Phenylalanine, and Fatty Acid Production
In 2020, the United Nations estimated that 2.37 billion people globally were without food or unable to eat a healthy balanced diet. The number of people with insufficient nutrition has increased in the short term due to COVID-19 pandemic and longer-term climate change is leading to shifts in arable land and water availability leading to a continued need to develop scalable sources of nutrition. One of the options that can yield high food mass per square foot of land use is the high-density culture of microalgae or other photosynthetic microorganisms. While photosynthetic microorganisms may provide high amounts of biomass with a small land footprint, the nutritional value of unmodified microorganisms may be limited. This mini-review presents the base nutritional value in terms of macro- and micronutrients of several cyanobacteria (Nostoc, Anabaena, Spirulina) in relation to established human nutritional requirements as a starting point for better utilization of cyanobacteria as nutritional supplements. It also discusses synthetic biology approaches that have been implemented in different organisms to increase the production of L-valine, L-phenylalanine, and fatty acids demonstrating some common genetic engineering design approaches and some approaches that are organism-specific.