Found 2 results
Open Access
Commentary
12 June 2024Dual Genetic Tracing Reveals the Origin of Alveolar Stem Cells after Lung Injury
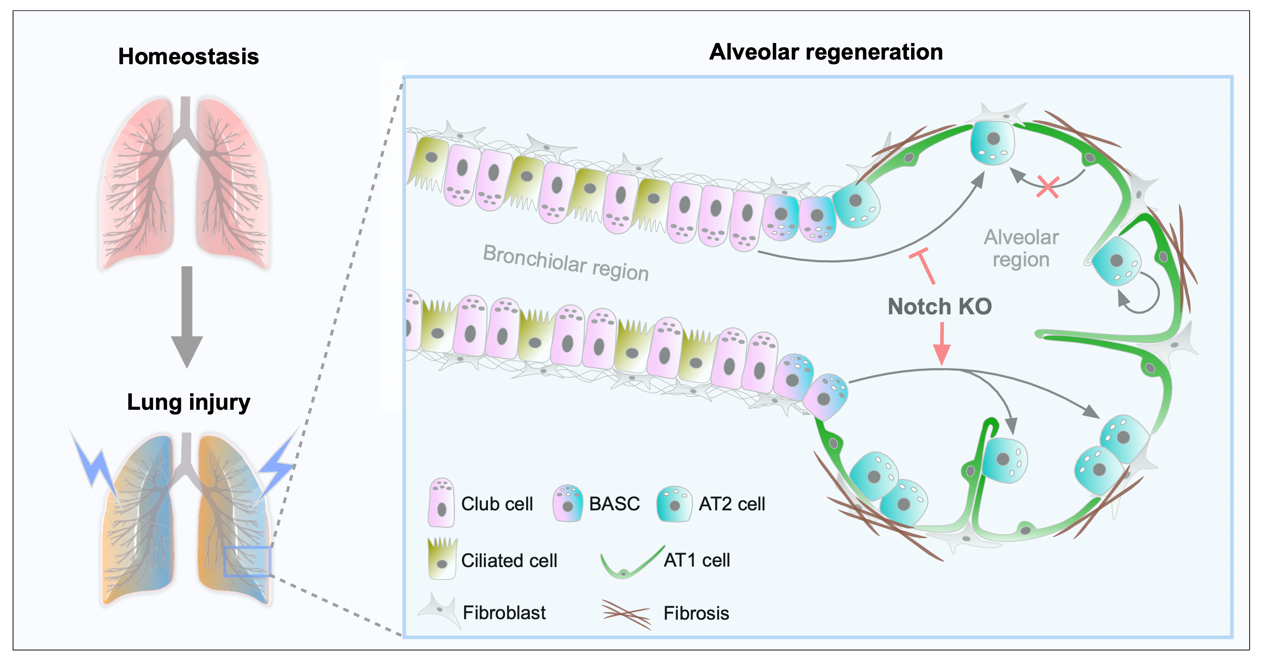
As alveolar epithelial stem cells, alveolar type II (AT2) cells play a pivotal role in sustaining alveolar homeostasis and facilitating repair processes. However, the sources of AT2 cell regeneration have remained contentious due to the non-specific labeling limitations of traditional single recombinase-based lineage tracing techniques. To address this issue, we employed dual recombination systems to develop more precise lineage tracing methodologies, effectively bypassing the shortcomings of conventional approaches and enabling specific labeling of lung epithelial cells. Our findings demonstrate that, following lung injury, regenerated AT2 cells do not originate from alveolar type I (AT1) cells, but instead derive from bronchiolar club cells and bronchioalveolar stem cells (BASCs), alongside the self-renewal of resident AT2 cells. Furthermore, we discovered that the transition of club cells and BASCs into AT2 cells is distinctly modulated by the Notch signaling pathway. This study not only provides novel insights into lung regeneration, but the innovative lineage tracing technology developed herein also holds promise as a technical support for research in diverse fields.
Open Access
Article
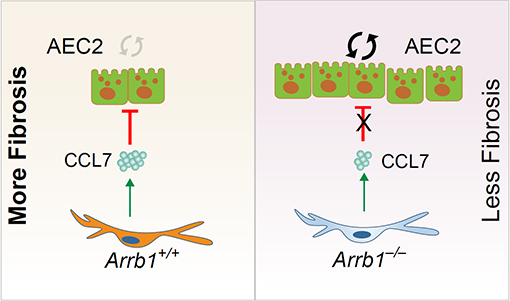
30 April 2024Arrestin beta 1 Regulates Alveolar Progenitor Renewal and Lung Fibrosis
The molecular mechanisms that regulate progressive pulmonary fibrosis remain poorly understood. Type 2 alveolar epithelial cells (AEC2s) function as adult stem cells in the lung. We previously showed that there is a loss of AEC2s and a failure of AEC2 renewal in the lungs of idiopathic pulmonary fibrosis (IPF) patients. We also reported that beta-arrestins are the key regulators of fibroblast invasion, and beta-arrestin 1 and 2 deficient mice exhibit decreased mortality, decreased matrix deposition, and increased lung function in bleomycin-induced lung fibrosis. However, the role of beta-arrestins in AEC2 regeneration is unclear. In this study, we investigated the role and mechanism of Arrestin beta 1 (ARRB1) in AEC2 renewal and in lung fibrosis. We used conventional deletion as well as cell type-specific deletion of ARRB1 in mice and found that Arrb1 deficiency in fibroblasts protects mice from lung fibrosis, and the knockout mice exhibit enhanced AEC2 regeneration in vivo, suggesting a role of fibroblast-derived ARRB1 in AEC2 renewal. We further found that Arrb1-deficient fibroblasts promotes AEC2 renewal in 3D organoid assays. Mechanistically, we found that CCL7 is among the top downregulated cytokines in Arrb1 deficient fibroblasts and CCL7 inhibits AEC2 regeneration in 3D organoid experiments. Therefore, fibroblast ARRB1 mediates AEC2 renewal, possibly by releasing chemokine CCL7, leading to fibrosis in the lung.