Sustainable Bioplastic Using Lignin Extracted from Neolamarckia cadamba Bark by Deep Eutectic Solvent
Received: 04 September 2025 Revised: 09 October 2025 Accepted: 10 November 2025 Published: 28 November 2025
© 2025 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
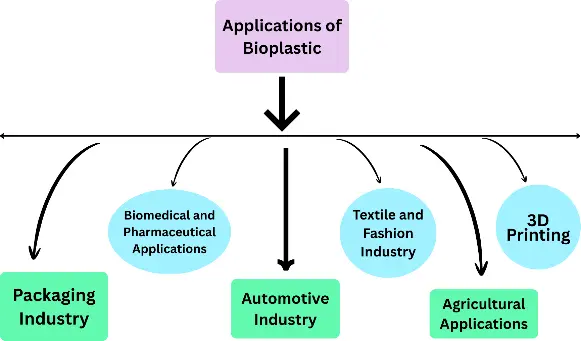
Lignocellulosic biomass, such as agricultural residues and forest byproducts, presents a sustainable and low-cost feedstock for producing various polymers without competing with food crops [1]. Advanced pretreatment techniques (e.g., steam explosion, ionic liquids, deep eutectic solvents) improve sugar yields and enable efficient fermentation and polymerisation routes for bioplastic synthesis [2]. Continuous innovation in biopolymer blends and composites is enhancing material performance, thereby expanding the applicability of bioplastics in sectors that demand higher strength, thermal stability, or biodegradability in diverse environmental conditions [3]. Bioplastics are a vast business, offering a sustainable alternative to plastic and having various applications, as shown in Figure 1.
Lignin is a complex structure that forms a strong backbone of plant walls (Figure 2) [4]. It is the second most abundant natural polymer after cellulose, accounting for 15–30% dry weight of a plant [5]. This complex biopolymer prevents microbial threats, strengthens plants, and facilitates the transfer of water. Although lignin is often burned for energy as a byproduct of the pulp and paper industry, its importance in sustainable development is seen in extended carbon materials, biofuels and environmentally friendly adhesives. Despite the processing difficulties caused by its complex molecular structure, lignin’s ability is being unlocked by advanced biotechnology, opening the door to a more durable, circular economy.
Neolamarckia cadamba, which belongs to the Rubiaceae family, sometimes called kadamba or burflower tree, is a tropical hardwood species that grows quickly and is indigenous to South and Southeast Asia (Figure 2). The tree grows to a height of about 17 m and a breast height of 25 cm in nine years under excellent growing conditions, making it perfect for plantation forestry’s production of biomass, pulp, paper, and lumber. It has been adopted in agroforestry and forest rehabilitation systems due to its quick growth and adaptation to damaged soils, providing both ecological and financial benefits [6]. Important lignin biosynthesis genes, such as NcCSE and NcHCT, have been found and described in N. cadamba from a molecular and biotechnological standpoint, providing information about the formation of its cell wall and secondary growth during xylogenesis [7]. In line with the objectives for sustainable biomass consumption, these molecular discoveries lay the groundwork for possible genetic modification targeted at altering the lignin content or composition to improve saccharification, pulp production, or biopolymer extraction [8].
The bark of the Neolamarckia cadamba tree is significant because it contains a substantial amount of lignin, making it useful for biopolymers and bio-based materials. The Klason lignin content of A. cadamba bark normally ranges from 25.68 to 31.71%, which is higher than the lignin content in its wood (22.8–23.9%). In this 30% of 100 g of N. cadamba bark powder would comprise lignin [9]. The bark is a promising raw material for applications like bioplastics, composites, and other lignin-derived products because of its high lignin proportion and availability as forestry waste.
Colocasia esculenta (Figure 2), also known as taro or cocoyam, is a cost-effective source of starch in tropical countries because of its exceptionally high starch content, which is typically 70–80% dry weight in its corms [10]. Numerous extraction techniques have been investigated. Compared to conventional extractions based on ammonia or alkali, the freeze-thaw method enhanced yield and functional characteristics. This method produces starch with a concentration between 80.3% and 81.3%, as well as improved hydration capacity, swelling, and freeze-thaw stability [11]. The typical starch granule size is very small 0.5–4 µm, sometimes up to 6.6 µm, which contributes to its rapid digestibility, hypoallergenic characteristics, and suitability for special food and industrial applications [12]. The versatility of taro starch as a stabiliser, emulsifier, fat substitute, and filler in food, pharmaceutical, and packaging applications was mentioned. Also, it was presented that modified starches, such as resistant starch, further increase the starch’s usefulness in functional and nutraceutical applications.
A Thymol–Menthol Deep Eutectic Solvent is a hydrophobic [13], natural DES composed of equimolar amounts of thymol and menthol (Figure 2). Thymol–Menthol DES differs from traditional ionic liquids and organic solvents (such as ethanol) due to its hydrophobicity, low volatility, and thermal stability [14]. With almost 100% atom economy and few byproducts, DESs are less expensive, biodegradable, and easier to prepare than ionic liquids. Its hydrophobic nature enhances the selective solubilization of lignin and other nonpolar compounds, facilitating green extraction under mild conditions. The chemical formulas of both chemicals are present. It has been demonstrated that thymol and menthol-based hydrophobic DESs have cytocompatibility, antibacterial, and antioxidant qualities. They are non-toxic to human cell lines and have antibacterial activity with low minimal inhibitory concentrations and anticandidal effects [15]. In the present paper experimental investigations have been performed for the synthesis of bioplastic using lignin from biomass extracted using DES. Various parameters have been tested for the synthesized bioplastic.
2. Materials and Methods
2.1. Materials
The chemical employed was hydrochloric acid (~35%, Merck, Vikhroli, Mumbai, India, EMPLURA), which had a density of 1.18 g/cm3 and a molecular weight of 36.46 g/mol. Glacial acetic acid (≥99.7%, Merck, EMPARTA) had a density of 1.049 g/cm3 and a molecular weight of 60.05 g/mol. The synthetic menthol (pure, SRL) (MW 156.27 g/mol) and thymol crystals (99%, SRL) (MW 150.22 g/mol) were used. Additionally, 98% AR grade, SRL sodium hydroxide pellets with a density of 2.13 g/cm3 and MW of 40.00 g/mol were employed. The summary of various reagents used is presented in Table 1.
Table 1. Necessary details of the chemicals used.
|
CAS Number |
Chemical Name |
Company (Brand) |
Purity |
Molecular Formula |
Molecular Weight |
Melting Point (°C) |
Boiling Point (°C) |
Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
|
7647-01-0 |
Hydrochloric Acid (~35%) |
Merck (EMPLURA) |
≥35% |
HCl |
36.46 |
114 (solution) |
110 (azeotrope) |
1.18 (35% sol.) |
|
64-19-7 |
Acetic Acid, Glacial (100%) |
Merck (EMPARTA) |
≥99.7% |
CH3COOH |
60.05 |
16.6 |
118 |
1.049 |
|
89-83-8 |
Thymol Crystal (99%) |
SRL (Sisco Research Laboratories) |
99% |
C10H14O |
150.22 |
49–51 |
232–233 |
0.96 |
|
2216-51-5 |
Menthol, Synthetic (pure) |
SRL (Sisco Research Laboratories) |
Pure |
C10H20O |
156.27 |
41–44 |
212 |
0.89 |
|
1310-73-2 |
Sodium Hydroxide Pellets |
SRL (Sisco Research Laboratories) |
98% |
NaOH |
40.00 |
318 |
Decomposes (>1388) |
2.13 (solid) |
2.2. Methodology
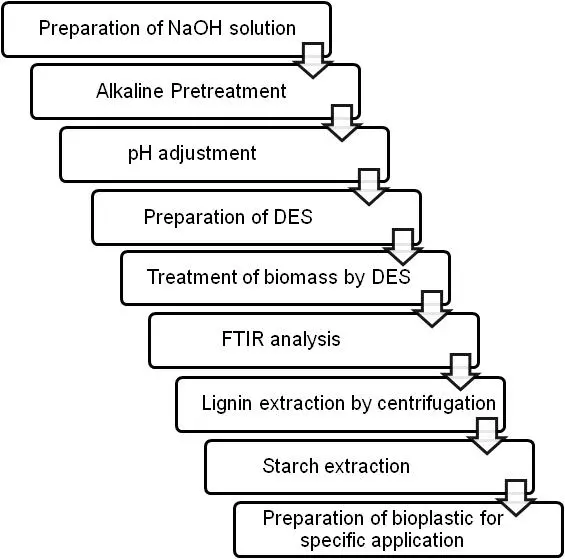
2.2.1. NaOH Solution Preparation and Alkaline Pretreatment Process
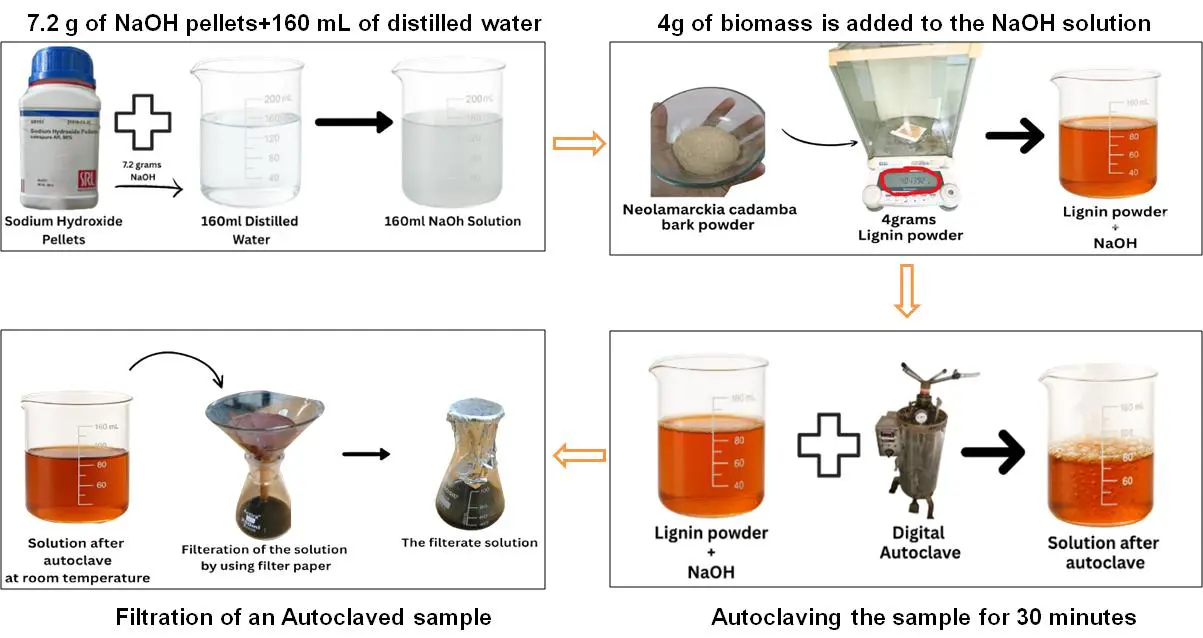
Sodium hydroxide (NaOH) aqueous solution of 0.3 N was prepared by accurately weighing 7.2 g of NaOH pellets on an analytical balance (Shimazdu, Kyoto, Japan, AUW220D) and dissolving them in 160 mL of distilled water (obtained from the distilled water unit, Merck KGaA, Darmstadt, Germany). Complete dissolution was ensured using a magnetic stirrer (Labquest, HLS 200). Subsequently, 4 g of biomass was mixed with a NaOH solution at a solid-to-liquid ratio of 1:20 (w/v). The resulting mixture was subjected to alkaline pretreatment under autoclave conditions at 121 °C and 15 psi for 30 min (Figure 4) [2,4].
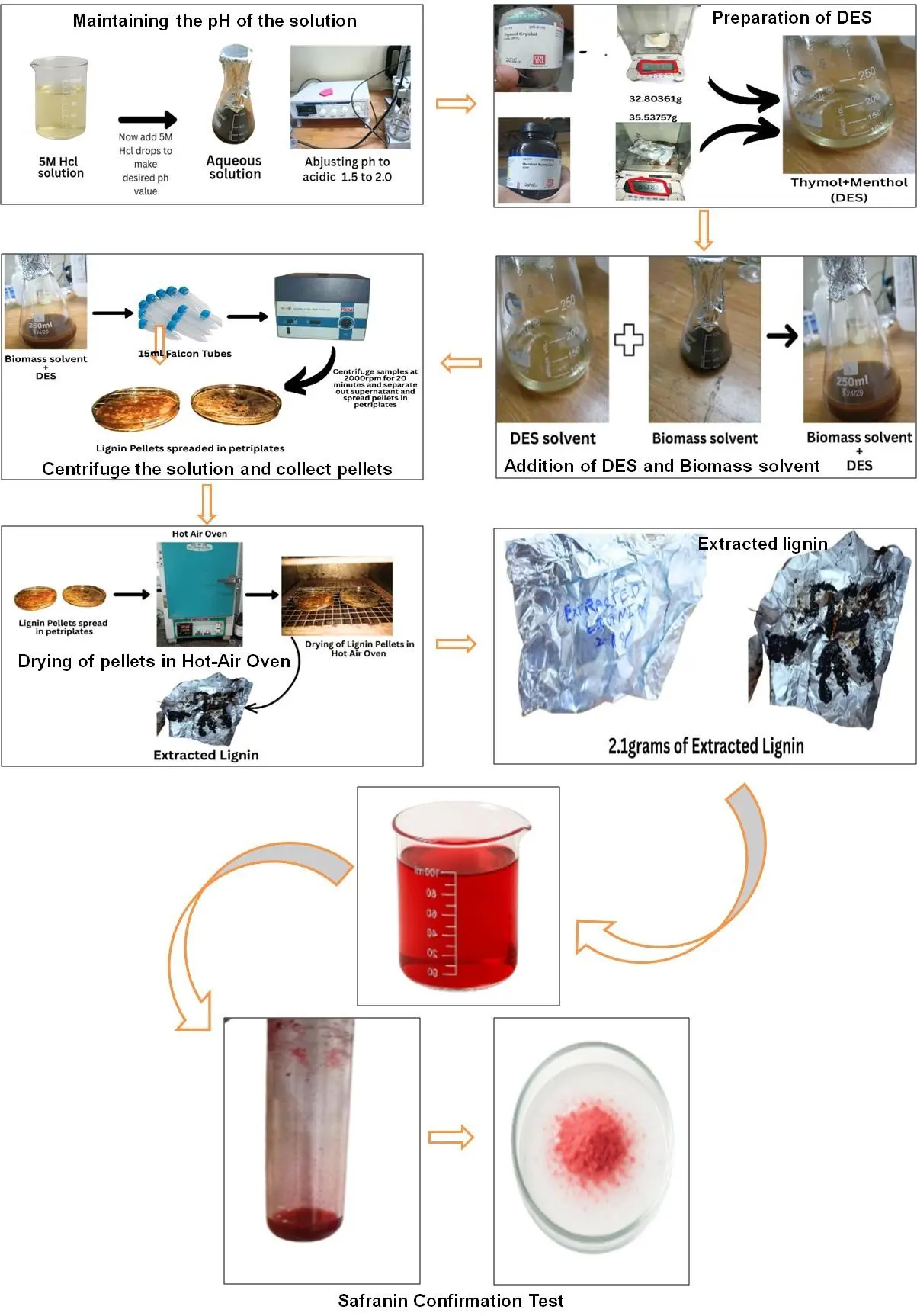
2.2.2. Preparation of DES, Lignin Extraction from Biomass via DES and Safranin Test Process
Thymol (CAS 89 83 8, C10H14O, MW 150.22 g/mol, purity 99 %, SRL Cat. 47110) and menthol synthetic (CAS 2216 51 5, C10H20O, MW 156.27 g/mol, SRL Cat. 64370) were precisely weighed and gently heated (~50–60 °C) until a clear, homogeneous liquid formed. The resulting solvent was cooled and stored at ambient conditions (8–25 °C), yielding a stable, low-melting liquid suitable for biomass extraction.
After alkaline pretreatment of biomass, the mixture was filtered through a sterile muslin cloth into a conical flask to separate the liquid phase from solid residues (Figure 5). The filtrate (35 mL) was obtained and used for lignin recovery. The extraction performance was estimated based on the recovery of lignin. The pH was measured (Labman, LMPH10H) and adjusted to 2.0 as per process requirement [2,4,13,14].
DES is added to the sample in a 3:1 ratio. The mixture was centrifuged at 2000 rpm for 20 min, and the separated materials were poured into Petri plates. These were kept in a hot-air oven for drying at 50–55 °C until complete removal of residual moisture was ensured. After drying, the lignin powders were gently scraped from the containers, weighed, and transferred into clean, dry, and labeled vials. For confirmation of lignin presence, the Safranin test was performed in which 100 mL of 0.5% aqueous safranin solution was prepared, and a few drops of it were poured on 0.1 g of lignin extracted, and then the lignin’s brown colour changed into reddish-pink colour, which proves lignin’s presence. UV-spectroscopy is also performed to confirm lignin’s presence at 280 nm (Figure 5) [2,4,13,14,15].
2.2.3. Starch Extraction and Preparation of Bioplastic
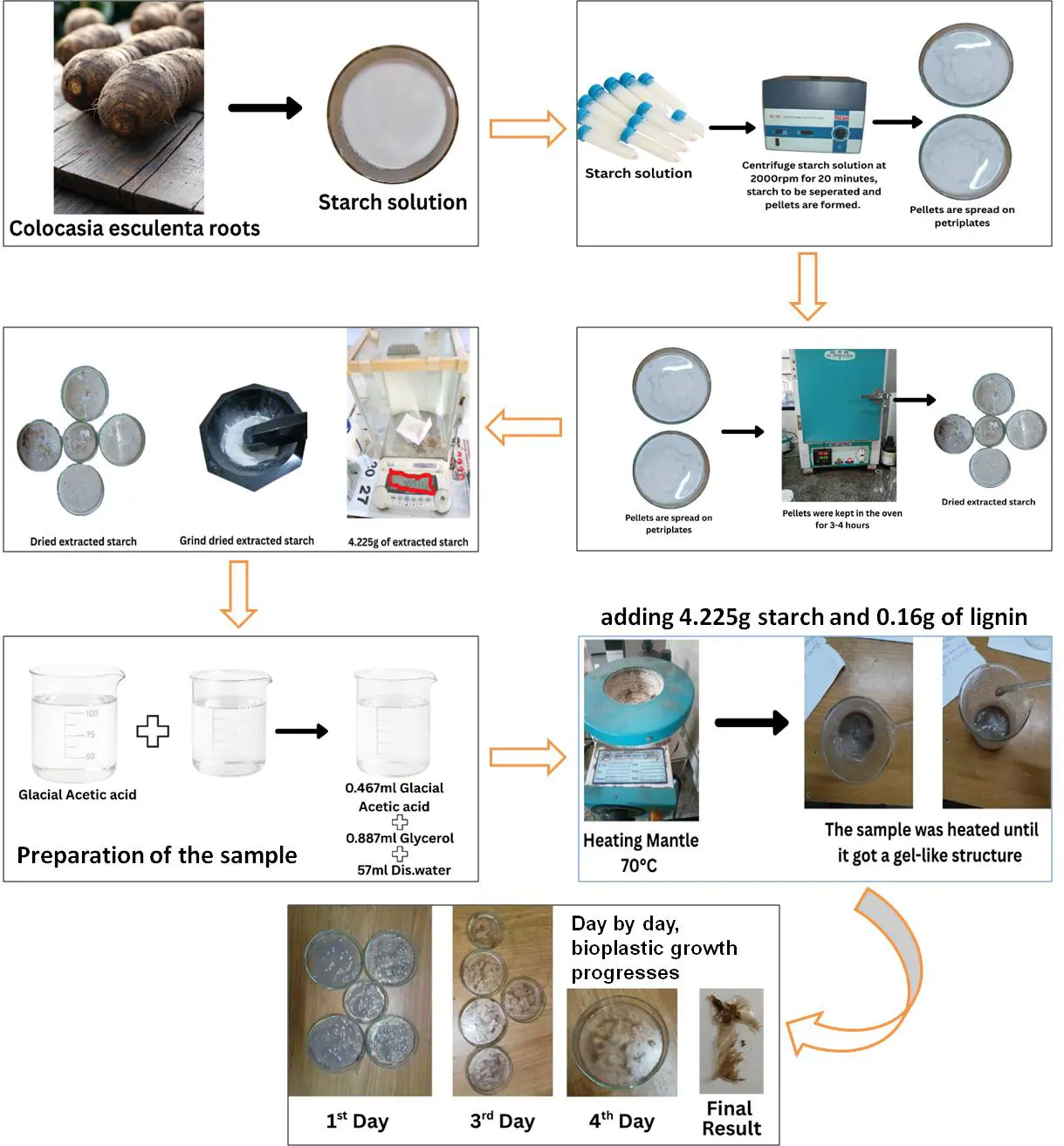
Freshly harvested Colocasia roots were washed thoroughly with tap water to remove adhering soil particles, followed by a rinse with distilled water. The roots were peeled, chopped into small sections, and ground into a slurry using a blender with distilled water in a 1:2 (w/v) ratio. The homogenized mixture was filtered through a muslin cloth to separate coarse fibers, and the filtrate was collected. The filtrate was allowed to stand undisturbed at room temperature for 4–6 h to facilitate starch precipitation. The clear supernatant was carefully decanted, and the starch-rich sediment was washed repeatedly with distilled water until the wash water appeared transparent. The collected sediment was then dried in a hot air oven at 40–50 °C until a constant weight was obtained. The dried starch was finely ground with a mortar and pestle and preserved in airtight containers for subsequent analysis (Figure 6) [16].
To produce bioplastic films, 4.225 g of starch powder and 0.16 g lignin were suspended in 50 mL of distilled water with continuous stirring to obtain a homogeneous mixture. Glycerol (0.887 mL) was incorporated as a plasticizer, and a small volume of acetic acid (0.467 mL) was added to facilitate gelatinization and enhance film formation. The suspension was heated on a hot plate at 70–80 °C (Biotechnics India, Mumbai, India, CXT-10) with constant agitation until it developed into a transparent, viscous gel. The hot gel was poured onto a flat glass plate (or Petri dish) and uniformly spread to form a thin film. The cast film was left to dry either at ambient temperature (for 4–5 days) or in a hot air oven (Biotechnics India, DTC-908) at 40–50 °C until the water content was completely removed. Once dried, the resulting bioplastic film was carefully detached and stored in airtight containers for subsequent testing and analysis [16].
2.2.4. Bioplastics Tests
For the prepared bioplastic, the moisture content, water solubility, and soil biodegradation were estimated, and the procedure has been presented in Figure 7 [17].
Moisture Content Determination
In this test, 0.3 g of bioplastic was placed in a hot air oven at 105 °C for 24 h, and then the difference between the initial and final weights was observed, from which the moisture content in the bioplastic was calculated.
Water Solubility Test
For water solubility, 0.471 g of bioplastic was dissolved in distilled water, and then it was kept in a hot air oven at 25 °C for 24 h. The final solution was filtered and the bioplastic was again kept in the oven to dry for 30 min. The difference between the initial weight and the final weight is observed to estimate water solubility.
Soil Biodegradation Test
For confirmation of the soil biodegradation test, 0.218 g of bioplastic was kept in the soil for 7 days. The difference between the initial and final weights of the bioplastic is measured to determine the percentage of biodegradation.
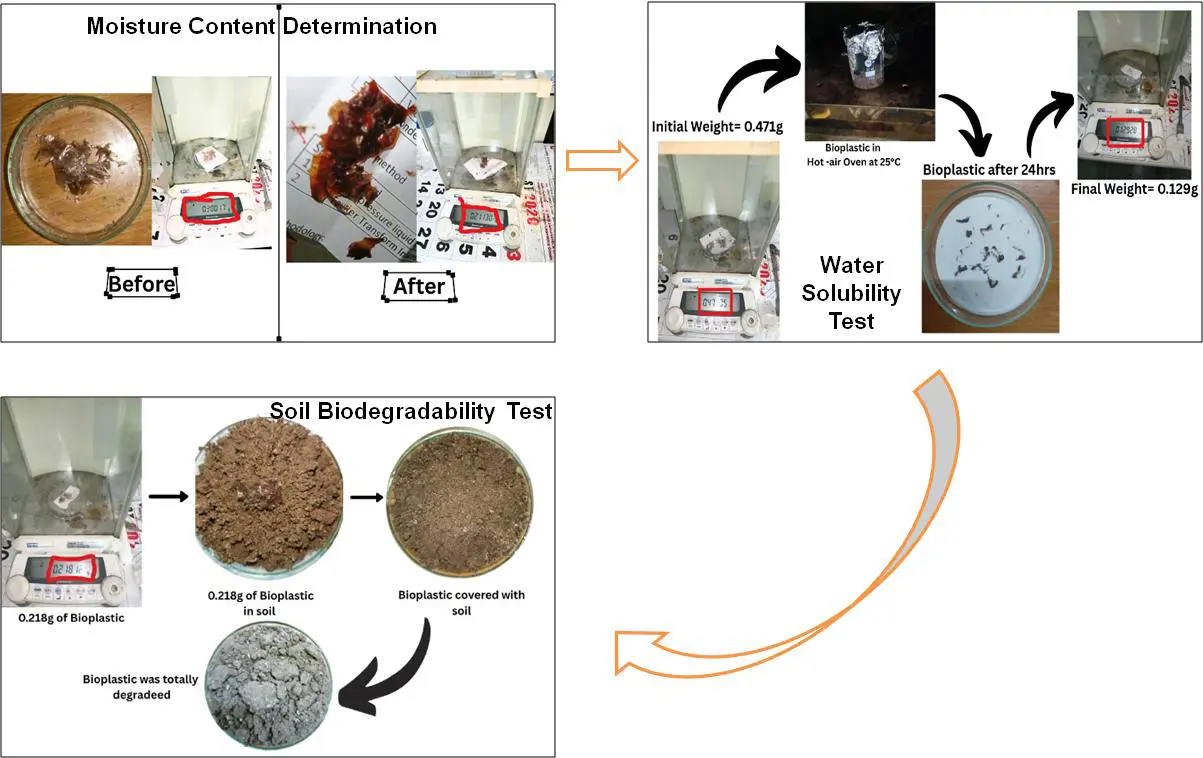
Figure 7. Moisture content, water solubility, and soil biodegradation procedure for bioplastic test.
3. Results and Discussion
3.1. Lignin Extraction
Lignin was extracted using thymol–menthol DES with yield of 52.5% (2.1 g of lignin recovered from 4.0 g of biomass powder). The improved yield was observed due to the hydrophobic character and robust hydrogen-bonding network of thymol–menthol DES which demonstrated well solublization of lignin. The recovered lignin showed up as a reddish-brown amorphous solid. Safranin staining, a qualitative test, was used to further validate its authenticity. The presence of aromatic and phenolic compounds was suggested by the emergence of a reddish-pink hue. These results further confirmed the successful extraction of lignin.
3.2. FTIR Spectroscopic Analysis of Extracted Lignin
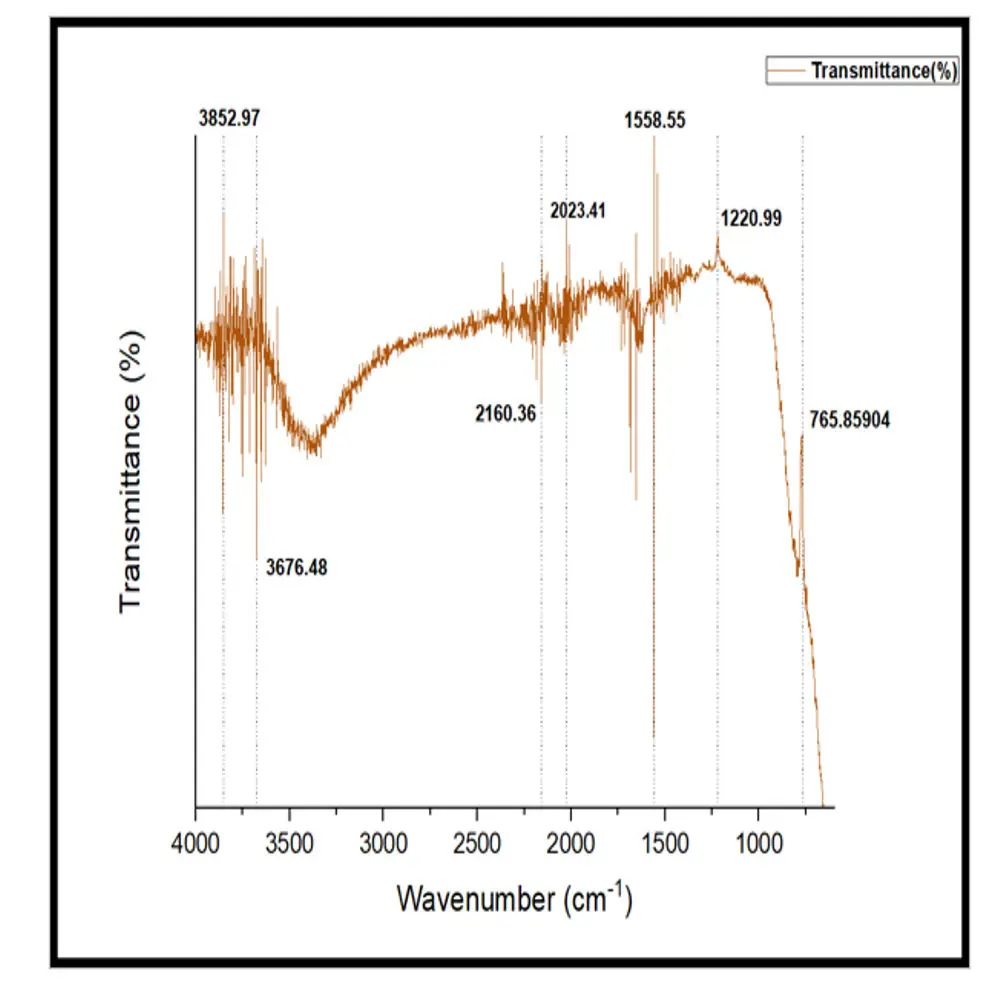
Fourier-Transform Infrared (FTIR) spectroscopy (Shimazdu, IRAffinity-1) was used to characterize the chemical structure and confirm the identity of lignin extracted from Neolamarckia cadamba bark as shown in Figure 8. The FTIR spectrum obtained displays a series of peaks representing different vibrational modes of functional groups that are typically associated with lignin’s complex aromatic and polyphenolic structure (Figure 8). Peaks observed in the 3852–3676 cm−1 region indicated O–H stretching (free and bonded), while strong absorption at 1558.55 cm−1 (aromatic C=C stretching) confirmed the preservation of the lignin aromatic backbone. Additional peaks at 1220.99 cm−1 (C–O deformation of phenolic/ether groups) and 765.86 cm−1 (aromatic C–H out-of-plane bending) further confirmed the signature functionalities of lignin. These results validated both the chemical identity and structural preservation of the extracted lignin. The FTIR spectrum of the extracted lignin spans a range from 4000 cm−1 to 600 cm−1, and it shows multiple transmittance dips at specific wavenumbers. These correspond to the vibrational modes of chemical bonds present in the extracted lignin. The most prominent regions and their implications are presented in Table 2.
Table 2. Fourier-Transform Infrared (FTIR) spectroscopy table.
|
Peak (cm‒1) |
Functional Group |
Interpretation |
Relative Intensity |
|---|---|---|---|
|
3852.97 |
O–H stretching (free) |
Free hydroxyls in phenolic/aliphatic compounds |
Medium |
|
3676.48 |
O–H stretching (H-bonded) |
Hydrogen-bonded hydroxyl groups in lignin or DES |
Strong |
|
2160.36 |
Possibly C≡N/overtone |
Minor trace or overtone band (aromatic system) |
Weak |
|
2023.41 |
Aromatic overtone/conjugation |
Aromatic π-system related overtone or matrix effect |
Weak |
|
1558.55 |
C=C stretching (aromatic ring) |
Aromatic ring stretch in guaiacyl/syringyl units (lignin) |
Very Strong |
|
1220.99 |
C–O stretch (aryl ether) |
Ether linkage in guaiacyl/syringyl lignin subunits |
Strong |
|
765.86 |
Aromatic C–H out-of-plane bend |
Substituted benzene rings—typical lignin signal |
Medium |
The FTIR analysis of the extracted lignin reveals characteristic absorption bands consistent with established lignin signatures: broad peaks in the 3400–3700 cm−1 region correspond to O–H stretching, indicating both free and hydrogen-bonded hydroxyl groups typical of phenolic and aliphatic alcohols in lignin [16]. The band observed at around 1550–1600 cm−1, specifically at ~1558 cm−1, corresponds to the aromatic C=C skeletal stretching, a primary indicator of lignin’s aromatic ring structure [17]. The absorption near 1220 cm−1 is attributed to C–O stretching in phenolic and ether linkages, including syringyl and guaiacyl units commonly found in lignin monomers [18]. Finally, the band near 765–850 cm−1 corresponds to aromatic C-H out-of-plane bending, confirming substituted aromatic ring structures typical of lignin polymers [19].
3.3. UV-Visible Spectroscopic Analysis of Lignin Extracted by DES
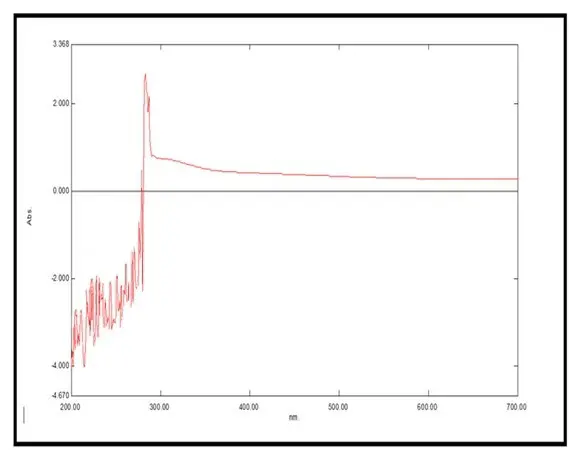
To confirm the aromatic and phenolic structure of the lignin extracted from Neolamarckia cadamba bark, UV-Visible (UV-Vis) spectroscopy (Shimazdu, UV-1800) was employed as shown in Figure 9. The spectral scan was recorded over the wavelength range of 200 to 700 nm, targeting both the ultraviolet and visible light regions, which are highly sensitive to the presence of conjugated π-electron systems-particularly the phenolic and aromatic structures characteristic of lignin. The resulting spectrum showed a strong and well-defined absorbance maximum at approximately 280 nm [20]. This absorption band is a hallmark of lignin and is widely reported in literature as being associated with π → π* electronic transitions occurring in aromatic rings and phenolic hydroxyl groups [21]. The presence of this peak strongly supports the successful extraction of lignin and, more importantly, confirms the retention of its conjugated aromatic backbone. In the context of this project, this is especially significant as the lignin was isolated using a natural, hydrophobic Deep Eutectic Solvent (DES) based on thymol and menthol, and one of the central objectives was to validate whether this eco-friendly extraction technique could maintain the structural integrity of lignin. The strong 280 nm peak also indirectly validates that the thymol-menthol DES system is not overly aggressive or depolymerizing-unlike harsh alkaline or acidic methods-which would typically shift or weaken this absorbance due to structural degradation of aromatic domains. This finding aligns with the FTIR data in the report, where a strong C=C stretching vibration at 1558.55 cm−1 similarly confirmed preservation of lignin’s aromatic ring structure.
In addition to the principal peak, the spectrum showed notable baseline noise and instability in the 200–270 nm range, which may be attributed to several factors:
-
-
Light scattering from particulate matter is not completely removed during filtration.
-
-
Residual background absorbance from DES components (particularly thymol or menthol) that are known to absorb in the low UV region.
-
-
Inadequate blank correction, as the DES solvent itself should ideally be used as the blank reference to eliminate matrix interference.
Despite this low-wavelength interference, the region beyond 300 nm remained largely featureless, suggesting the absence of additional coloured impurities, and further reinforcing the selective extraction of lignin as the primary UV-active component in the sample. From a functional perspective, the confirmation of intact aromatic and phenolic domains through this UV-Vis analysis is highly relevant to downstream application-i.e., the formulation of bioplastic composites. Aromatic rings in lignin contribute not only to UV resistance and antioxidant potential but also act as cross-linking sites for enhanced mechanical strength in lignin–starch biopolymer films. This directly complements the thermal and solubility properties observed in the bioplastic characterisation section, where the inclusion of lignin was found to contribute to reduced water solubility and improved structural robustness. Additionally, when coupled with the safranin staining results-which visually confirmed the presence of phenolics through reddish-pink colouration and FTIR spectroscopy, the UV-Vis data provides a tripartite confirmation of both the identity and purity of the extracted lignin.
3.4. Synthesis of Lignin-Based Bioplastic
Following lignin isolation, a biodegradable plastic was synthesised by blending extracted lignin (0.1 g) with starch (4.225 g) extracted from Colocasia esculenta roots. A plasticising agent glycerol (0.887 mL) was used along with a mild acid catalyst 5% glacial acetic acid (prepared by diluting 5 mL of glacial acetic acid in 95 mL of distilled water). These components were suspended in 57.2 mL of distilled water to form a working solution. This mixture was heated at 70 °C under constant stirring until a gel-like structure formed. The gel was cast into Petri dishes and allowed to air-dry over four days, producing flexible bioplastic films.
3.5. Bioplastic Characterization Tests
To evaluate the performance and applicability of the produced bioplastic, several standard characterization tests were carried out. The moisture content was obtained as 29.59%, which is a relatively high moisture content typical for starch-based films and contributes to flexibility but may reduce shelf life. The water solubility was estimated experimentally as 72.61% which indicates that the hydrophilic nature and suitability of the polymer for applications where water solubility is favorable, such as temporary packaging or agricultural films. A biodegradability test was performed in soil for seven days, and almost complete (98%) degradation was observed after recovery and drying. This confirms the capacity for degradation under natural conditions, making it suitable for environmentally responsible disposal.
4. Conclusions
Lignin was successfully extracted from Neolamarckia cadamba bark using a hydrophobic Deep Eutectic Solvent (DES) composed of thymol and menthol. The green solvent system demonstrated excellent extraction efficiency while avoiding the use of toxic or environmentally harmful reagents. The extracted lignin was characterised through multiple analytical techniques, including UV-Vis spectroscopy, FTIR, and visual staining with safranin. The prominent absorbance peak at ~280 nm in the UV-Vis spectrum confirmed the presence of aromatic and phenolic structures, while FTIR analysis further validated the retention of lignin’s characteristic functional groups.
The results highlight that the thymol–menthol DES preserved the structural integrity of lignin’s aromatic backbone, making it suitable for downstream applications in biomaterial development. The reddish-pink colouration observed with safranin staining visually reinforced the presence of phenolic compounds, supporting the spectral findings [22]. Collectively, these analyses confirm that the lignin extracted via DES is chemically viable and sufficiently pure for incorporation into sustainable products such as lignin-based bioplastics.
Overall, this experiment demonstrates that hydrophobic DESs like thymol-menthol can be employed as effective, and eco-friendly alternatives for lignin extraction from lignocellulosic biomass. The success of this approach not only adds value to agro-industrial waste but also supports the broader goals of green chemistry and circular bioeconomy [23]. By this, numerous approaches can be taken, as seen in this experiment, where bioplastic was prepared for use in preparing biofuels, medicines, and to help lead a sustainable life [24]. Future work may focus on scaling the extraction process, improving lignin purification, and exploring different bioremediation techniques.
Statement of the Use of Generative AI and AI-Assisted Technologies in the Writing Process
During the preparation of this manuscript, the author(s) used QuillBot to improve the clarity and readability of certain sections of the text. QuillBot was used solely as a language enhancement tool and not as a substitute for the author(s)’ own interpretation, analysis, or scientific reasoning. After using QuillBot, the author(s) thoroughly reviewed, verified all content to ensure accuracy, originality, and compliance with ethical publication standards. The author(s) take full responsibility for the integrity and final quality of the published article.
Author Contributions
H.R.—Methodology, Formal Analysis, validation, Writing Original Draft Preparation. A.T.—Conceptualization, Data curation, writing—review & editing, D.S.—Conceptualization, Supervision, Project Administration, R.C.—Conceptualization, Supervision, Project Administration, K.W.—Conceptualization, Supervision, Project Administration.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
Al-Battashi HS, Annamalai N, Sivakumar N, Al-Bahry S, Tripathi BN, Nguyen QD, et al. Lignocellulosic biomass (LCB): A potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev. Environ. Sci. Bio/Technol. 2019, 18, 183–205. doi:10.1007/s11157-018-09488-4. [Google Scholar]
-
Alawad I, Ibrahim H. Pretreatment of agricultural lignocellulosic biomass for fermentable sugar: Opportunities, challenges, and future trends. Biomass Convers. Biorefin. 2022, 14, 6155–6183. doi:10.1007/s13399-022-02981-5. [Google Scholar]
-
Nanda S, Patra BR, Patel R, Bakos J, Dalai AK. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2021, 20, 379–395. doi:10.1007/s10311-021-01334-4. [Google Scholar]
-
Singh SK, Dhepe PL. Isolation of lignin by organosolv process from different varieties of rice husk: Understanding their physical and chemical properties. Bioresour. Technol. 2016, 221, 310–317. doi:10.1016/j.biortech.2016.09.042. [Google Scholar]
-
Ververis C, Georghiou K, Christodoulakis N, Santas P, Santas R. Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production. Ind. Crops Prod. 2004, 19, 245–254. doi:10.1016/j.indcrop.2003.10.006. [Google Scholar]
-
Balan S, Ramalingam G, Selvakumar D, Adhimoolam K, Jayakodi M, Arunachalam B, et al. A review of Kadamba (Neolamarckia cadamba): An invaluable medicinal plant. Genet. Resour. Crop Evol. 2025, 72, 5093–5113. doi:10.1007/s10722-025-02329-8. [Google Scholar]
-
Li J, Huang X, Huang H, Huo H, Nguyen CD, Pian R, et al. Cloning and characterization of the lignin biosynthesis genes NcCSE and NcHCT from Neolamarckia cadamba. AMB Express 2019, 9, 152. doi:10.1186/s13568-019-0860-z. [Google Scholar]
-
Mishra A, Mishra TK, Nanda S, Mohanty MK, Dash M. A comprehensive review on genetic modification of plant cell wall for improved saccharification efficiency. Mol. Biol. Rep. 2023, 50, 10509–10524. doi:10.1007/s11033-023-08886-4. [Google Scholar]
-
Arisandi R, Ihda FV, Nirsatmanto A, Sunarti S, Lukmandaru G, Tsuchikawa S, et al. Characterization of Chemical Components in the Wood and Bark of Anthocephalus cadamba and Anthocephalus macrophyllus from Progeny Trial in Wonogiri, Indonesia. BIO Web Conf. 2025, 167, 06001. doi:10.1051/bioconf/202516706001. [Google Scholar]
-
Kaushal P, Kumar V, Sharma HK. Utilization of taro (Colocasia esculenta): A review. J. Food Sci. Technol. 2013, 52, 27–40. doi:10.1007/s13197-013-0933-y. [Google Scholar]
-
Majzoobi M, Farahnaky A. Granular cold-water swelling starch; properties, preparation and applications, a review. Food Hydrocoll. 2021, 111, 106393. doi:10.1016/j.foodhyd.2020.106393. [Google Scholar]
-
Ranathunga RAA, Suwannaporn P. Young cereal grains as a new source of healthy and hypoallergenic foods: A review. J. Food Sci. Technol. 2021, 59, 3336–3348. doi:10.1007/s13197-021-05228-9. [Google Scholar]
-
Van Osch DJ, Dietz CH, Van Spronsen J, Kroon MC, Gallucci F, van Sint Annaland M, et al. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. doi:10.1021/acssuschemeng.8b03520. [Google Scholar]
-
Bagović Kolić M, Železnjak M, Markov K, Gaurina Srček V, Cvjetko Bubalo M, Radošević K, et al. Physicochemical and Biological Properties of Menthol and Thymol-Based Natural Deep Eutectic Solvents. Molecules 2025, 30, 1713. doi:10.3390/molecules30081713. [Google Scholar]
-
Bedair HM, Samir TM, Mansour FR. Antibacterial and antifungal activities of natural deep eutectic solvents. Appl. Microbiol. Biotechnol. 2024, 108, 198. doi:10.1007/s00253-024-13044-2. [Google Scholar]
-
Sammons RJ, Harper DP, Labbé N, Bozell JJ, Elder T, Rials TG. Characterisation of Organosolv Lignins using Thermal and FT-IR Spectroscopic Analysis. BioResources 2013, 8, 2752–2767. DOI:10.15376/biores.8.2.2752-2767. [Google Scholar]
-
Huang Y, Wang L, Chao Y, Nawawi DS, Akiyama T, Yokoyama T, et al. Analysis of Lignin Aromatic Structure in Wood Based on the IR Spectrum. J. Wood Chem. Technol. 2012, 32, 294–303. doi:10.1080/02773813.2012.666316. [Google Scholar]
-
Zhuang J, Li M, Pu Y, Ragauskas A, Yoo C. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. doi:10.3390/app10124345. [Google Scholar]
-
Barsberg S, Matousek P, Towrie M. Structural Analysis of Lignin by Resonance Raman Spectroscopy. Macromol. Biosci. 2005, 5, 743–752. doi:10.1002/mabi.200500042. [Google Scholar]
-
Zhang Y, Naebe M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorbe. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. doi:10.1021/acssuschemeng.0c06998. [Google Scholar]
-
Yang J, Ching Y, Chuah C. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. doi:10.3390/polym11050751. [Google Scholar]
-
Baldacci-Cresp F, Spriet C, Twyffels L, Blervacq A, Neutelings G, Baucher M, et al. A rapid and quantitative safranin-based fluorescent microscopy method to evaluate cell wall lignification. Plant J. 2020, 102, 1074–1089. doi:10.1111/tpj.14675. [Google Scholar]
-
Weligama Thuppahige VT, Moghaddam L, Welsh ZG, Wang T, Xiao HW, Karim A. Extraction and Characterisation of starch from cassava agro-industrial waste. LWT 2023, 182, 114787. doi:10.1016/j.lwt.2023.114787. [Google Scholar]
-
Folino A, Pangallo D, Calabrò PS. Assessing Bioplastics biodegradability by std and research methods: Current trends & open issues. J. Environ. Chem. Eng. 2023, 11, 109424. doi:10.1016/j.jece.2023.109424. [Google Scholar]