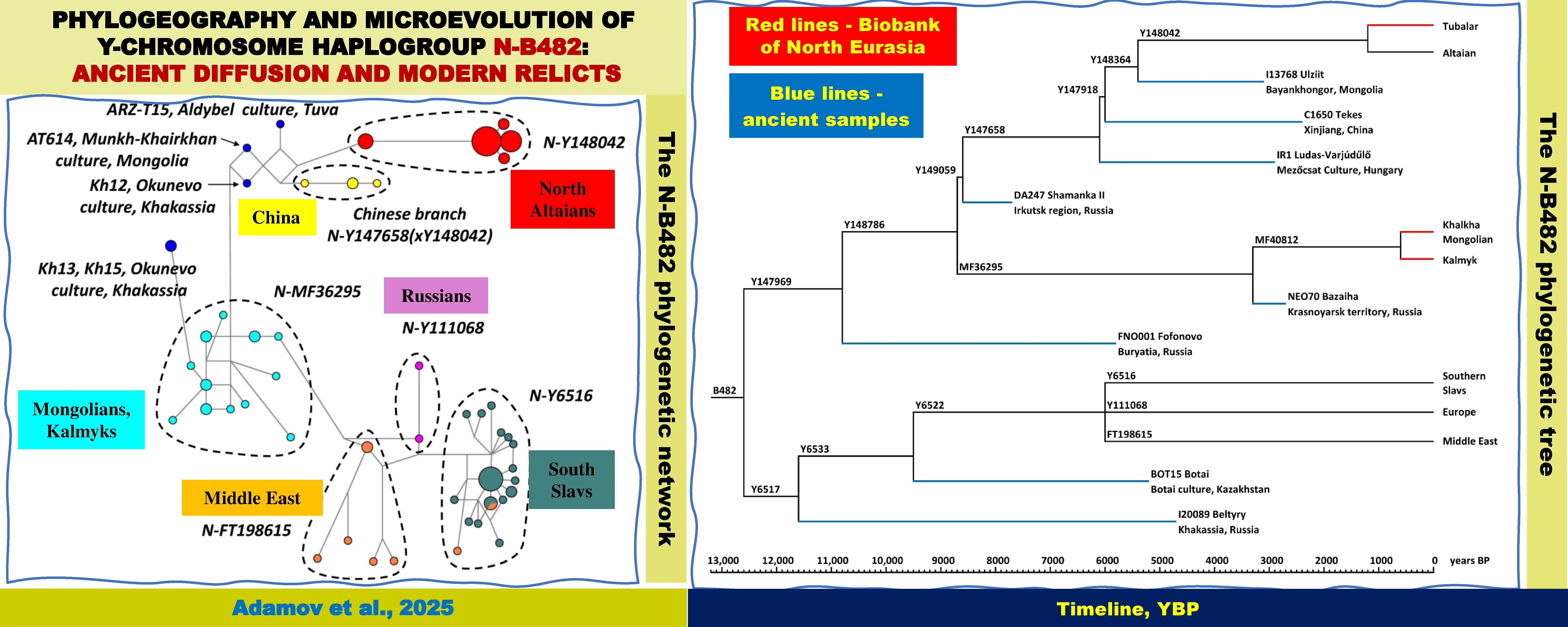
Phylogeography and Microevolution of Y-Chromosome Haplogroup N-B482: Ancient Diffusion and Modern Relicts
Received: 16 September 2025 Revised: 15 October 2025 Accepted: 21 October 2025 Published: 28 October 2025
© 2025 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
The advent of whole-genome sequencing, its rapid evolution, and the ensuing vast collections of genetic data have opened the door to exploring the genetic history of human populations, providing us with the tools to study differences in human DNA nucleotide sequences. The time of genetic events can be estimated using probabilistic mathematical models that treat mutations as a molecular clock. Single-nucleotide polymorphisms (SNPs) in the Y-chromosome create a variation between the Y-chromosomes of the male progenitor and his male offspring, which in future generations translates into the growth of new branches (haplogroups) on the world’s human Y-chromosome phylogenetic tree, varying in their abundance and time of emergence. Another type of mutation, known as short tandem repeats (STRs), gives rise to Y-STR haplotypes that differ from one another in the number of repetitive nucleotide sequences, referred to as motifs. The probability of STR mutations (i.e., the probability of a higher or lower number of motifs) is much higher than the probability of SNP mutations; therefore, the rates of STR and SNP mutations in the “molecular clock” are compared to the movements of the second and hour hands, respectively.
In this publication, we analyze STR and SNP mutations to study the phylogenetic structure of the haplogroup N-B482. The N-B482 was primarily discovered in a single sample in [1]. Since then, no studies have described this haplogroup. It is a small branch of N-M231, one of 20 major human Y-chromosome haplogroups [2]. The other branch of N-M231, called N-Z4762, is common in modern populations of Eurasia. According to YFull (https://www.yfull.com/tree/, accessed on 9 August 2025), N-B482, also known as N-Y6503, has two subhaplogroups that diverged ~12,800 YBP, namely N-Y147969 represented in the YFull database by a single modern sample from the Republic of Altai, Russia. The other, N-P189.2, is found in a few dozen commercial samples, most of which come from the Balkans [3]. The N-P189.2 was discovered during commercial testing and appeared on the ISOGG tree in 2012. However, its position remained uncertain until the publication of a paper by Ilumae et al. (2016) [1]. The haplogroup N-B482 first appeared on the ISOGG tree in the 2017 version. Due to a lack of information, even the latest version of ISOGG 2019–2020 does not include the N-Y147969. According to the literature, subhaplogroup N-Y147969 is found in Northern Altaians at 6% to 12% [4,5,6]. The complete Y-chromosome sequence of a modern Altaian (ERS2478500) is provided in [7].
It is well known that the number of family names passed down through male descendants grows smaller over time. In nations with a long tradition of family names, such as Han or Korean, there are relatively few modern family names. This phenomenon was first studied in the 19th century in France (Bienaymé) and the UK (Galton, Watson) in connection with the mass extinction of aristocratic family names. Genetics considers a family name similar to a genetic marker correlated most distinctly with the Y-chromosome of men [8]. The extinction of family names and Y-chromosome variants is observed not only within individual lineages but also within entire Y-chromosome haplogroups. Even the earliest studies of human Y-chromosome phylogeny reported a high rate of extinction of Y-chromosome lineages [9].
This publication presents the first comprehensive study of the rare haplogroup N-B482, based on the analysis of Y-SNP markers, Y-STR markers, and Y-chromosome whole-genome sequences from modern population samples provided by the Biobank of North Eurasia, as well as ancient genomes from the existing literature. The integrated approach allowed us to estimate the distribution of N-B482 in the past and present, update its phylogenetic structure, and assess factors that had once caused a sharp reduction in the number of its carriers.
2. Materials and Methods
The samples of modern populations were provided by the Biobank of North Eurasia [10]. The samples had been previously collected following approval by the Ethics Committee of the Bochkov Centre for Medical Genetics and the acquisition of informed donor consents. We analyzed samples of unrelated male individuals who are third-generation descendants of men born in and identifying as members of the studied population. DNA was isolated from venous blood by phenol-chloroform extraction using proteinase K. Y-chromosome SNP markers were genotyped by real-time PCR with TaqMan probes (Applied Biosystems, Waltham, MA, USA) using a 7900 HT Fast Real-Time PCR System (Applied Biosystems, USA) and an OpenArray technology (Applied Biosystems) on a QuantStudio 12K Flex System (Applied Biosystems, USA). Fragment analysis was carried out using an ABI 3130xl Genetic Analyzer (Applied Biosystems) and a Yfiler PCR Amplification Kit (Applied Biosystems) for 17 Y-STR loci.
The median-joining algorithm [11] was applied to construct phylogenetic networks of 17 Y-STR (Yfiler) haplotypes within haplogroup N-B482. For each marker, its weight was assumed to be 10, and ε was set to 0. Median networks were reconstructed in Network v.10.2.0.0; images for the networks were prepared in Network Publisher v.2.1.2.5. The time to the most recent common ancestor (TMRCA) for the identified phylogenetic network clusters was estimated with the ASD method [12]. The mutation rate in the analyzed 17-Y-STR haplotypes (0.0026 mutations per locus per generation) was derived from YHDR records [13]. The average male generation time was assumed at 31.5 years [14].
Library preparation for three samples (Altai Tubalar, Kalmyk, and Khalkha Mongolian) and their whole-genome sequencing were conducted at the Evogen Medical Genetics Laboratory (Evogen, Moscow, Russia). Working DNA concentrations were determined using a Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). DNA purity was assessed from optical density readings using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, USA). DNA libraries were prepared following the PCR-free protocol provided by the MGIEasy Fast PCR-FREE FS Library Prep Set V2 manufacturer (MGI, Shenzhen, China). The protocol was preceded by enzymatic fragmentation on a MGISP-960 automated sample preparation workstation (MGI, China). Quality checks were conducted as per the manufacturer’s protocol. The samples were sequenced on a DNBSEQ-T7 platform (MGI, China) in the PE150 mode. The average read depth was >30×, with Q30 > 85%.
The applied single nucleotide variant (SNV) detection protocol for genomic data consisted of raw data pre-processing, read mapping to the reference genome, duplicate marking, and variant calling. Briefly, adapter sequences and low-quality reads were removed using the Cutadapt utility (version 4.6, https://cutadapt.readthedocs.io/en/v4.6/, accessed on 7 May 2025). The filtered reads were mapped to the reference human genome GRCh38 (hg38) using BWA (version 0.7.17, https://github.com/lh3/bwa/releases, accessed on 7 May 2025). SAM to BAM conversion was done in SAMtools (version 1.7, https://www.htslib.org/, accessed on 7 May 2025), the BAM files were then sorted and indexed. Duplicate reads were located and tagged by GATK MarkDuplicatesSpark (version 4.3.0.0, https://github.com/broadinstitute/gatk/releases, accessed on 7 May 2025); VCF files were generated using DeepVariant (version 1.4.0, https://github.com/google/deepvariant/releases, accessed on 7 May 2025).
Among the detected SNVs, SNP mutations were identified using a method by Adamov et al. [15], which effectively discriminates true mutations from reading, mapping, and other errors. TMRCA was estimated from the number of mutations in the diverged branches within specific Y-chromosome segments (“combBED” regions) totaling 8.47 Mbp in length. Within these regions, SNP mutations can be reliably identified because combBEDs primarily contain euchromatin X-degenerated nucleotide sequences. The rate constant of SNP mutations was assumed to be 0.82 × 10−9 per nucleotide per year [15,16].
3. Results
3.1. Gene Geography of Haplogroup N-B482 in the Modern Population
The vast collection of the Biobank of North Eurasia [10] comprises over 25,000 DNA samples from more than 100 indigenous populations of North Eurasia. These samples are genotyped for a broad range of Y-SNP markers, with a significant proportion also genotyped for 17 or 37 Y-STR markers. For the purpose of this study, the entire array of the Biobank’s samples was screened for Y-STR haplotypes; the screening revealed that 30 samples belonged to carriers of different N-B482 lineages.
The haplogroup N-B482, also known as N-Y6503, is divided into two subhaplogroups that diverged ~12,800 YBP: the Altaian subhaplogroup N-Y147969 and the Balkan subhaplogroup N-P189.2 [3].
Of 30 Biobank’s samples with Y-STR haplotypes representing haplogroup N-B482, 29 belonged to the Altaian subhaplogroup N-Y147969, including 12 Altai Tubalars, 8 Altai Kumandins, 5 Altai Chelkans, 2 Kalmyks, and 2 Khalkha Mongolians. The Balkan subhaplogroup N-P189.2 was represented by one Russian from the Pskov region. To increase the sample size, we searched for 17 Y-STR haplotypes in the literature and YHRD [13]. The search brought up a few haplotypes that fell into the Altaian subhaplogroup N-Y147969, including 3 Tubalars and 1 Chelkan from [6], 1 Tubalar from [17], and 1 Altaian sample (from an unspecified population) retrieved from the whole-genome data provided in [7]. We also found 4 published N-Y147969 haplotypes from China and 12 from different Mongolian populations. Thus, our pooled dataset for the Altaian subhaplogroup N-Y147969 comprised 51 Y-STR haplotypes. The majority of N-Y147969 carriers (n = 31) in the dataset were North Altaians from small indigenous populations settled in the Republic of Altai, Altai territory, and Kemerovo region, including Kumandins, Chelkans, and Tubalars (~7300 individuals in total). They traditionally dwell in remote, poorly accessible areas of the Altai Mountains northwest of Lake Teletskoye. Their ethnogenesis is linked to the long history of contact between autochthonous Ugric, Samoyedic, and probably Yeniseian (Ket) tribes and migrations of Turkic-speaking populations [18,19,20].
The Balkan subhaplogroup N-P189.2, detected in modern European populations, is also rare. Its highest frequency (3–4%) is observed in Serbs [21,22,23] and does not exceed 1% in Croatia, Slovenia, Bosnia and Herzegovina [24,25,26]. In addition to one Russian sample from the Biobank’s collection, our dataset of 17 Y-STR haplotypes of the subhaplogroup N-P189.2 included 36 samples from YHRD [13].
In total, the final dataset of 17-Y-STR haplotypes of haplogroup N-B482 included 88 modern samples. A summary of these samples is provided in Supplementary Table S2.
3.2. Phylogenetic Structure of Haplogroup N-B482
3.2.1. North Altaian Branch of Altaian Subhaplogroup N-Y147969
The analysis of two whole genomes representing subhaplogroup N-Y147969 (an Altai Tubalar and an Altaian from [7]) placed each of the samples within a separate lineage of this North Altaian subhaplogroup. The number of repeats for their modal (the most frequent) Y-STR haplotypes is presented below in the ascending order of numerical designations for Y-STR loci, i.e., as DYS19, DYS385a, DYS385b, DYS389I, DYS389II, DYS390, DYS391, DYS392, DYS393, DYS437, DYS438, DYS439, DYS448, DYS456, DYS458, DYS635, GATA-H4.
Repeats in the first lineage (N-GG1092): 17-11-13-14-31-24-10-14-14-15-9.2-11-20-17-17-23-11;
Repeats in the second lineage (N-Y148261) 16-11-13-14-30-24-10-14-14-15-9.2-11-20-16-17-21-11.
The first lineage, which we named N-GG1092, was represented by the Tubalar, marked by the C→T mutation at position 9031355 and included 27 samples from the pooled dataset of North Altaians (n = 31). The second lineage N-Y148261, was represented by the remaining four samples (3 Kumandins and 1 Altaian from an unspecified population from [7]). The count of SNP mutations in the whole genomes of the Tubalar and the Altaian placed divergence of N-GG1092 and N-Y148261 at 1220 ± 300 YBP.
3.2.2. Chinese Branch of Altaian Subhaplogroup N-Y147969
In both lineages, locus DYS438 contains an extremely rare allelic microvariant 9.2. We found 4 more samples carrying this microvariant allele in Chinese populations, which constitute a separate Chinese branch:
16-11-13-13-31-24-10-14-13-15-9.2-11-21-15-17-21-12,
16-11-13-13-31-24-10-14-13-15-9.2-11-21-15-17-21-12,
16-11-13-13-31-24-10-14-13-15-9.2-11-21-15-17-21-13,
16-11-13-14-31-24-10-14-13-15-9.2-11-21-15-17-21-12.
The first three samples were retrieved from YHRD [13], where they were described as Chinese Hans; the fourth sample belonged to a Tibetan from the Gansu province [27]. The phylogenetic analysis (Figure 1) of Y-STR haplotypes suggested a relatively close relationship between the North Altaian and Chinese lineages. The ASD interclade estimate of TMRCA was 5400 ± 1900 YBP, placing the Chinese branch within subhaplogroup N-Y147969.
Knowing the observed frequency of the samples representing the subhaplogroup N-Y147969 in our pooled dataset and assuming that the male to female ratio in the general population was 1:1, we estimated the number of N-Y147969 carriers at 400 men for its North Altaian branch and at 20,000 men for the Chinese branch, concluding that both branches are relicts.
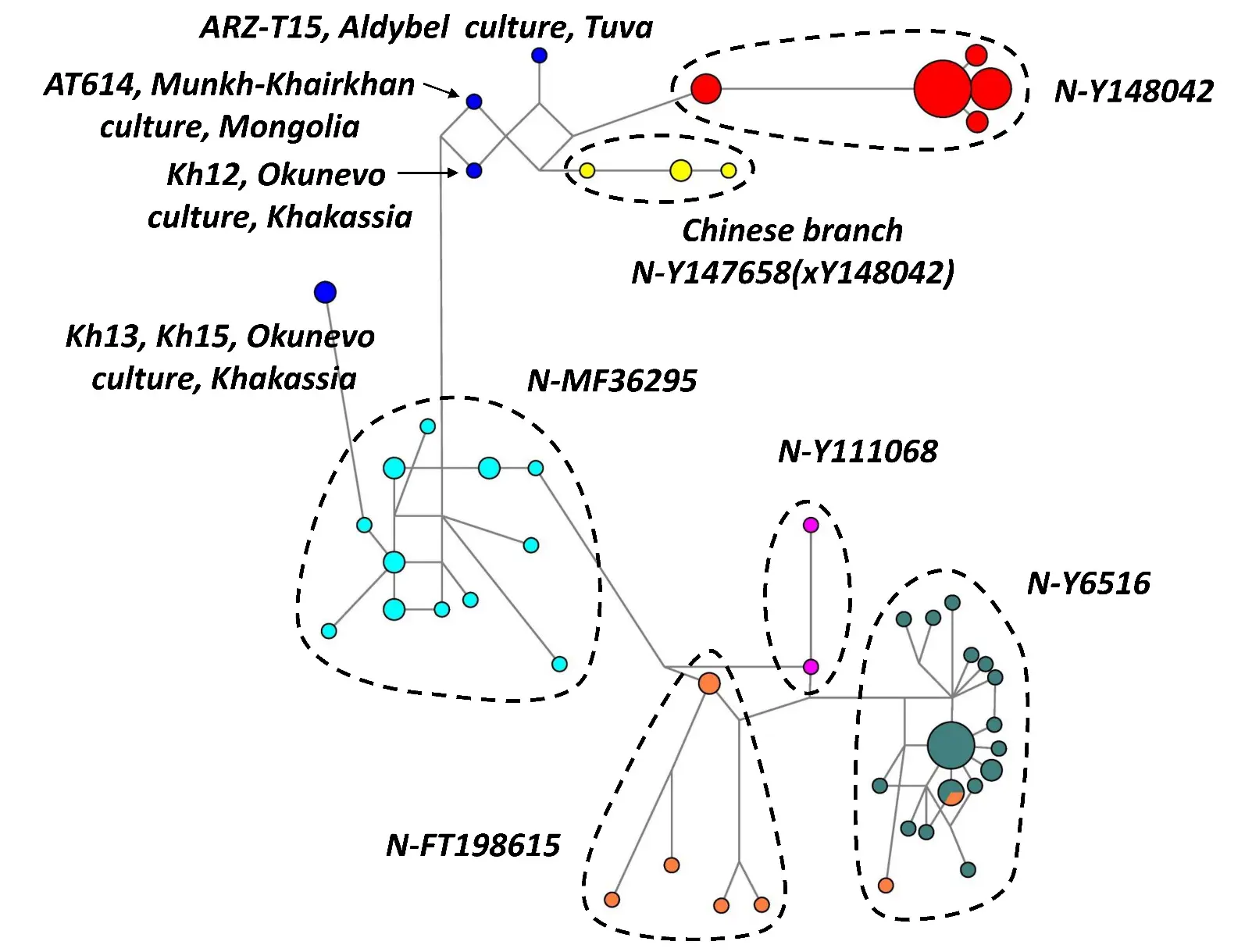
Figure 1. A phylogenetic network for 17 Y-STR haplotypes of the haplogroup N-B482 (n = 93). Color codes for haplotypes: ancient are shown in blue, North Altaians in red, samples from China in yellow, Mongolians and Kalmyks in aqua, South Slavs in dark cyan, Middle East in light orange, Russians in fuchsia color.
3.2.3. Mongolian Branch of Altaian Subhaplogroup N-Y147969
While screening the Biobank’s collection for Y-STR haplotypes, we discovered another branch of N-Y147969 missing from the YFull and FTDNA phylogenetic trees. We named this branch Mongolian since its carriers were two Khalkha Mongolians (Mongolia) and two Kalmyks (Russia). The modal haplotype of this branch consists of the following tandem alleles listed in the ascending order of Y-STR loci:
15-11-14-13-31-24-10-15-14-15-10-13-20-14-18-21-12.
The search for Y-STR haplotypes in the literature and databases brought up 12 more samples of this rare Mongolian branch. Among its carriers, there were 3 Mongolians and 1 Daur from Hulunbuir (China) [28], 2 Kalmyks [29], and 3 Mongolians from different regions of Inner Mongolia (China) [30,31,32]. One more haplotype from Mongolia and two from China were found in YHRD [13]. According to the whole-genome analysis of the Khalkha Mongolian and Kalmyk samples, this branch is marked by the MF36295 mutation. The count of SNP mutations suggested that the Mongolian (N-MF36295) and the North Altaian (N-Y148042) branches diverged at 8700 ± 1000 YBP. TMRCA for the Mongolian branch estimated on the basis of 17 Y-STR markers is 1900 ± 600 YBP.
3.2.4. Balkan Subhaplogroup N-P189.2
Another subhaplogroup of N-B482 is N-P189.2. In modern samples, the allelic microvariant 24.3 at locus DYS390 simplifies the search for its carriers. The search for Y-STR haplotypes in YHRD [13] and the Biobank of North Eurasia brought up 2 major geographic regions of N-P189.2 presence: the Balkan Peninsula and the Middle East. The Balkan Peninsula was represented by a total of 27 samples from Serbia (n = 19), Bosnia and Herzegovina (n = 5), Croatia (n = 2), and Slovenia (n = 1). The Middle East was represented by a total of 7 samples from Kuwait (n = 2), Lebanon (n = 1), Qatar (n = 1), Saudi Arabia (n = 1), Iran (n = 1), and Turkey (n = 1). Two samples from Russia and one from the US also fell within the subhaplogroup N-P189.2. According to FTDNA (https://discover.familytreedna.com/y-dna/N-FGC28401/tree, accessed on 19 January 2025), N-P189.2 has 3 branches: N-Y6516, N-Y111068, and N-FT198615, but this information is not provided in the scientific literature. Knowing about N-P189.2 branches, we were able to place the retrieved haplotypes of South Slavs and the haplotypes from Saudi Arabia and Turkey within branch N-Y6516, locate N-FT198615 to the Middle East, and discover that N-Y111068 comprises rare haplotypes from Europe, including the Russian samples from the Ryazan [13,33] and Pskov regions (our sample from the Biobank of North Eurasia).
3.3. Search for Haplogroup N-B482 Carriers in the Ancient Population
A phylogenetic network was constructed for the pooled dataset of modern and ancient 17 Y-STR haplotypes of the haplogroup N-B482 (n = 93). Figure 1 shows that the haplotypes form distinct clusters corresponding to the branches of the Altaian subhaplogroup N-Y147969 (North Altaian branch N-Y148042, Chinese branch N-Y147658(xY148042), Mongolian branch N-MF36295) and the Balkan subhaplogroup N-P189.2 (branch N-Y6516 of South Slavs, the Middle East branch N-FT198615, and 2 Russian samples representing N-Y111068).
Today, all N-B482 branches are rare. However, the literature describes 28 ancient samples representing N-B482 and suggests that N-B482 was common in Eurasia in ancient times. Table 1 summarizes the published data on ancient samples dated to 8000–2000 YBP.
Table 1. Summarized data on ancient N-B482 samples in chronological order.
|
Sample |
Location |
Age YBP |
Archaeological Information |
Refs. |
Geno- Typing |
Branch |
|---|---|---|---|---|---|---|
|
011874 |
Zhokhov Island, Yakutia, Russia |
8000 |
Mesolithic |
[34] |
SNP |
N-P189.2 |
|
DA247 |
Shamanka II, Irkutsk region, Russia |
7787–7610 |
Early Neolithic |
[7] |
SNP |
N-Y147969 |
|
I12695 |
Razdum’ye-1, Altai territory, Russia |
7670–7600 |
Early Neolithic |
[35] |
SNP |
N-P189.2 |
|
I11107 |
Sosnovy-Mys, Irkutsk region, Russia |
8000–6000 |
Serov culture |
[35] |
SNP |
N-Y147969 |
|
FNO001 |
Fofonovo, Buryatia, Russia |
6000–5500 |
Neolithic |
[36] |
SNP |
N-Y147969 |
|
I15934 |
Kangalassy, Yakutia, Russia |
5488–5448 |
Syalakh-Belkachi complex |
[35] |
SNP |
N-Y147969 |
|
I13260 |
Tuzovskie-Bugry-1, Altai territory, Russia |
5600–5150 |
Late Neolithic-Eneolithic |
[35] |
SNP |
N-B482 |
|
BOT15 |
Botai, North Kazakhstan region, Kazakhstan |
5293–4976 |
Botai culture |
[7] |
SNP |
N-P189.2 |
|
I20089 |
Beltyry, Khakassia, Russia |
4822–4534 |
Afanasievo culture, late stage |
[37] |
SNP |
N-P189.2 |
|
Kh12 |
Uybat-Charkov, Khakassia, Russia |
4500–4200 |
Okunevo culture |
STR |
N-Y147969 |
|
|
Kh13 |
Itkol’ II, Khakassia, Russia |
4500–4200 |
Okunevo culture |
STR |
N-Y147969 |
|
|
Kh15 |
Itkol’ II, Khakassia, Russia |
4500–4200 |
Okunevo culture |
STR |
N-Y147969 |
|
|
I11735 |
Mereke, West Kazakhstan region, Kazakhstan |
4412–4249 |
Poltavka culture |
[40] |
SNP |
N-P189.2 |
|
I11737 |
Mereke, West Kazakhstan region, Kazakhstan |
4145–3982 |
Poltavka culture |
[40] |
SNP |
N-P189.2 |
|
TIP001 |
Tipki, Stavropol territory, Russia |
4142–3837 |
Lola culture |
[41] |
SNP |
N-P189.2 |
|
NEO900 |
Sjauke, Pavlodar region, Kazakhstan |
3886–3721 |
Elunino culture |
[42] |
SNP |
N-P189.2 |
|
I13173/ |
Munkh-Khairkhan, Khovd, Mongolia |
3829–3644 |
Munkh-Khairkhan culture |
SNP, STR |
N-Y147969 |
|
|
I13768/ |
Ulziit, Bayankhongor, Mongolia |
3160–2997 |
Late Bronze Age |
[43] |
SNP |
N-Y147969 |
|
I11926 |
Ciumai, Taraclia district, Republic of Moldova |
n.d. |
Bronze Age (presumably) |
[44] |
SNP |
N-P189.2 |
|
I1503/ IR1 |
Ludas-Varjúdűlő, Hungary |
2928–2784 |
PreScythian Mezőcsát Culture |
[45] |
SNP |
N-Y147969 |
|
NEO70/ |
Bazaiha, Krasnoyarsk territory, Russia |
2775–2728 |
Late Bronze Age |
SNP |
N-Y147969 |
|
|
ARZ-T15 |
Eerbek river, Tuva, Russia |
2800–2400 |
Aldy-Bel culture |
[47] |
STR |
N-Y147969 |
|
I10944/ |
Himera, Sicily, Italy |
2430 |
Ancient Greek colony, battle of Himera 480 BC |
[48] |
SNP |
N-Y147969 |
|
C1650 |
Tekes, Xinjiang, China |
2490–2350 |
Early Iron Age |
[49] |
SNP |
N-Y147969 |
|
C1668 |
Tekes, Xinjiang, China |
2300–2008 |
Early Iron Age |
[49] |
SNP |
N-Y147969 |
|
I10519/ |
Turgen 2, Turgen, Almaty region, Kazakhstan |
2300–2000 |
Tien Shan Wusun |
[50] |
SNP |
N-Y147969 |
|
L8630 |
Rabat, Surkhandarya region, Uzbekistan |
2150–1950 |
Kushan Empire period |
[51] |
SNP |
N-P189.2 |
|
C1664 |
Tekes, Xinjiang, China |
2094–1974 |
Early Iron Age |
[49] |
SNP |
N-Y147969 |
To better illustrate the results, we compiled a geographic map with ancient and modern samples, presented in Figure 2.
Of 28 ancient samples (Table 1), 24 were genotyped for SNP mutations at various coverage depending on the degree of DNA degradation. The sample AT614 (subhaplogroup N-Y147969) from the Munkh-Khairkhan culture burial site was genotyped for both SNP and STR polymorphisms. This allowed us to place two more ancient samples with STR haplotypes (Kh12 from the Okunevo culture and ARZ-T15 from the Aldy-Bel culture) within the subhaplogroup N-Y147969. These samples had the microvariant allele 9.2 at locus DYS438. In the phylogenetic network, two ancient samples from the Okunevo culture (Kh13 and Kh15) are adjacent to the Mongolian branch N-MF36295 (Figure 1). The phylogenetic network illustrates convincingly the correctness of placing five ancient Y-STR haplotypes (Table 2) within modern branches (Figure 1, haplotypes are shown in black).
Table 2. Y-STR haplotypes of 5 ancient samples representing haplogroup N-B482 presented in the order of numerical designations: DYS19, DYS385a, DYS385b, DYS389I, DYS389II, DYS390, DYS391, DYS392, DYS393, DYS437, DYS438, DYS439, DYS448, DYS456, DYS458, DYS635, GATA-H4
|
Sample |
Y-STR Haplotype |
References |
|---|---|---|
|
Khakassia, Okunevo culture, sample Kh12 |
16-11-13-14-31-24-10-14-14-15-9.2-11-21-14-17-22-12 |
[38] |
|
Mongolia, Khovd aimag, Munkh-Khairkhan culture, sample AT614 |
16-11-13-14-31-24-10-14-14-15-9.2-11-21-15-18-22-12 |
[38] |
|
Tuva, Aldy-Bel culture, sample ARZ-T15 |
16-11-13-13-29-24-10-14-14-15-9.2-11-21-15-17-22-12 |
[47] |
|
Khakassia, Okunevo culture, sample Kh13 |
15-11-13-13-31-24-11-14-14-14-10-12-21-14-18-21-12 |
[38] |
|
Khakassia, Okunevo culture, sample Kh15 |
15-11-13-13-31-24-11-X-14-14-10-12-21-14-18-21-12 |
[38] |
Note: Х—data not available.
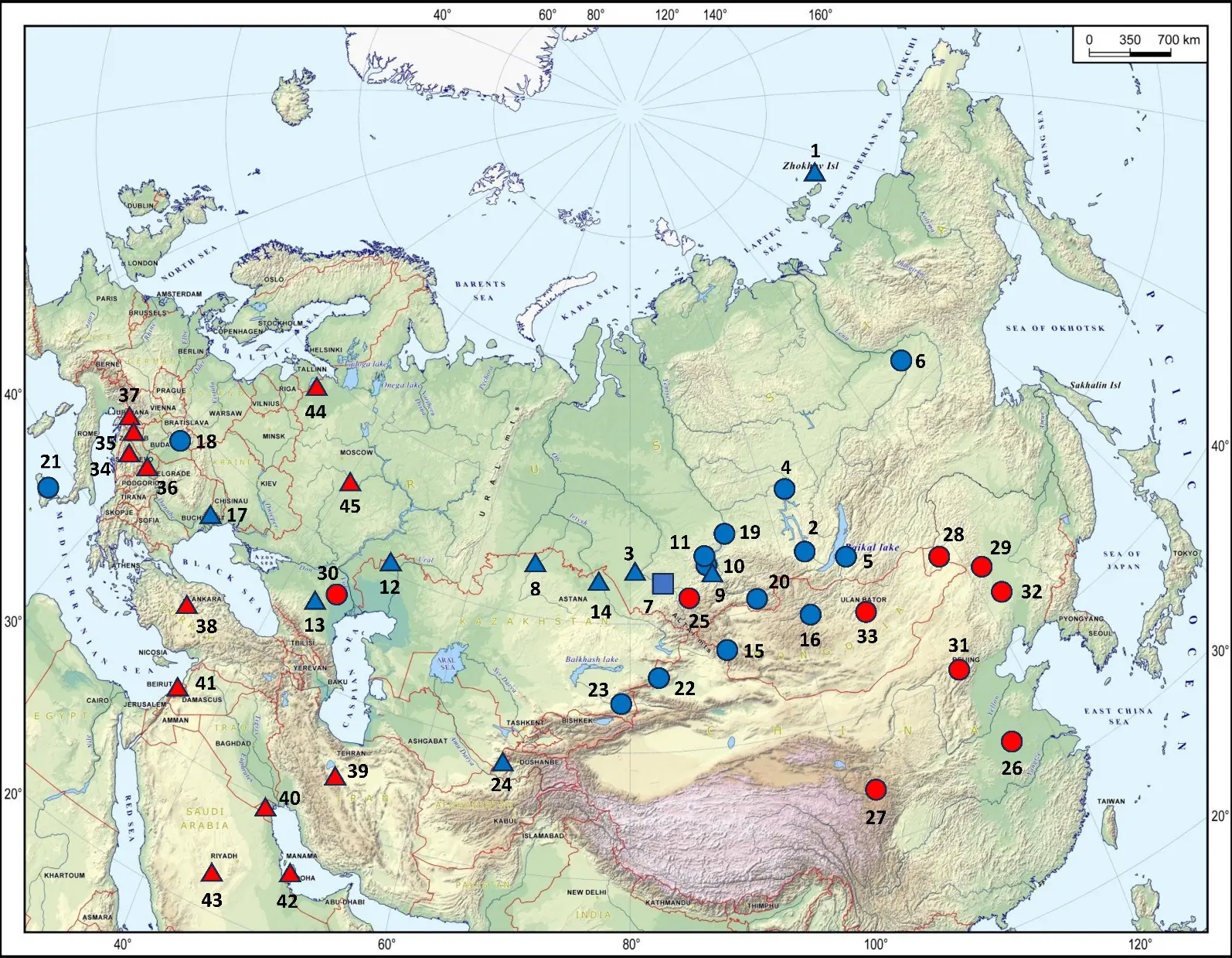
Figure 2. Geographical distribution of the N-B482 ancient and modern carriers. Color codes: ancient samples are shown in blue, modern in red. Legend symbols: circles—N-Y147969, triangles—N-P189.2, square—N-B482. Ancient samples: 1—Zhokhov Island, Yakutia, Russia; 2—Shamanka II, Irkutsk region, Russia; 3—Razdum’ye-1, Altai territory, Russia; 4—Sosnovy-Mys, Irkutsk region, Russia; 5—Fofonovo, Buryatia, Russia; 6—Kangalassy, Yakutia, Russia; 7—Tuzovskie-Bugry-1, Altai territory, Russia; 8—Botai, North Kazakhstan region, Kazakhstan; 9—Beltyry, Khakassia, Russia; 10—Uybat-Charkov, Khakassia, Russia; 11—Itkol’ II, Khakassia, Russia; 12—Mereke, West Kazakhstan region., Kazakhstan; 13—Tipki, Stavropol territory, Russia; 14—Sjauke, Pavlodar region, Kazakhstan; 15—Munkh-Khairkhan, Khovd, Mongolia; 16—Ulziit, Bayankhongor, Mongolia; 17—Ciumai, Taraclia district, Republic of Moldova; 18—Ludas-Varjúdűlő, Hungary; 19—Bazaiha, Krasnoyarsk territory, Russia; 20—Eerbek river, Tuva, Russia; 21—Himera, Sicily, Italy; 22—Tekes, Xinjiang, China; 23—Turgen 2, Turgen, Almaty region, Kazakhstan; 24—Rabat, Surkhandarya region, Uzbekistan. Modern samples: 25—Northern Altai, Russia; 26—China (no details found); 27—Gansu, China; 28—Hulunbuir, Inner Mongolia, China; 29—Jalaid Qi, Inner Mongolia, China; 30—Kalmykia, Russia; 31—Hohhot, Inner Mongolia, China; 32—Horqin district, Inner Mongolia, China; 33—Mongolia; 34—Bosnia and Herzegovina; 35—Croatia; 36—Serbia; 37—Slovenia; 38—Turkey; 39—Iran; 40—Kuwait; 41—Lebanon; 42—Qatar; 43—Saudi Arabia; 44—Pskov region, Russia; 45—Ryazan region, Russia.
3.4. Phylogenetic Analysis of Haplogroup N-B482 for Ancient and Modern Populations
Minding the hierarchy of SNP mutations in the genomic data from eight ancient and four modern samples, we constructed a phylogenetic tree for the haplogroup N-B482 (Figure 3). TMRCA for N-B482 samples was estimated based on two datasets. For the Altaian subhaplogroup N-Y147969, TMRCA was estimated from the average number of SNP mutations in four whole genomes (the Biobank’s samples of an Altai Tubalar, Kalmyk, Khalkha Mongolian, and an Altaian from [7]). For the Balkan subhaplogroup N-P189.2, the TMRCA was estimated based on the average number of mutations according to YFull (78 mutations after the branching point from a dataset of 44 samples) [3]. The accuracy of the estimates was good; TMRCA was dated to 12,600 ± 1300 YBP (information about SNP mutations in the combBED regions of the Altai Tubalar, Kalmyk, and Khalkha Mongolian samples is given in the Supplementary Table S1).
The obtained dating for the oldest branching point of haplogroup N-B482 was used as a timeline to date other branches of the phylogenetic tree. Briefly, we aligned the allelic reads from the ancient samples to the list of mutations detected in the modern samples of N-Y147969 and N-P189.2 carriers. The divergence time for these lineages was estimated from the ratio of the ancestral to the derived alleles in the ancient samples sequenced at high coverage (see time scale in Figure 3).
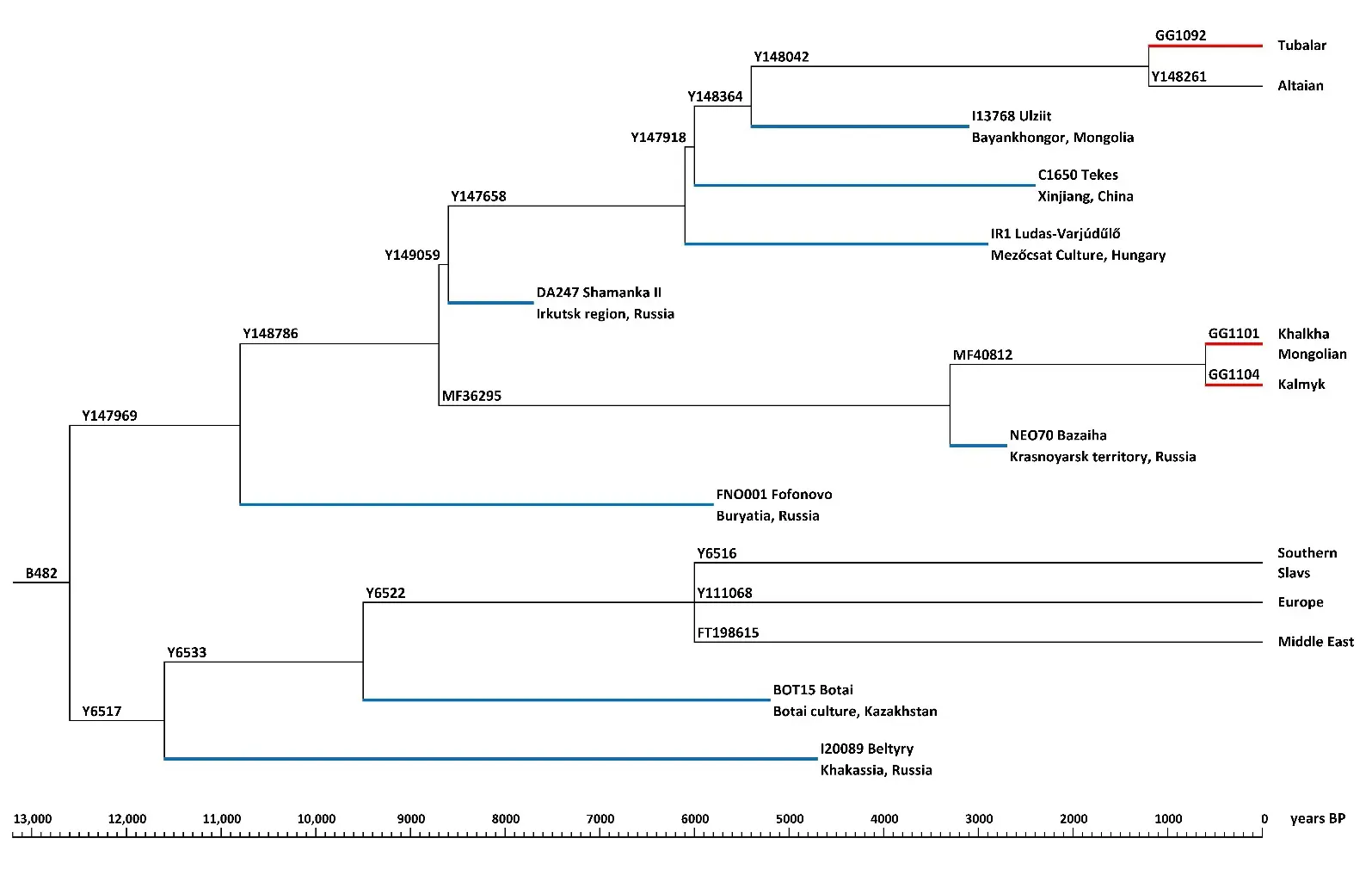
Figure 3. The phylogenetic tree of the haplogroup N-B482. Red lines represent branches of our whole genome sequencing samples, while blue lines represent branches of ancient samples. The x-axis represents the time scale in YBP.
We were unable to accurately identify the lineages of seven out of 13 ancient samples representing the Altaian subhaplogroup N-Y147969 because of low coverage. However, some of the identified mutations suggested with certainty the positions of those samples in the N-Y147969 phylogenetic tree (Figure 3). The haplogroup branch of the sample from the Fofonovo burial site (6000–5500 YBP) diverged from other samples at 10,800 ± 2500 YBP. The sample I11107 from the Irkutsk region (Sosnovy-Mys, 8000–6000 YBP) constituted a separate ancient lineage not found in modern samples. The ancient samples from Yakutia (Kangalassy, ~5500 YBP) and the Krasnoyarsk territory (Bazaiha, ~2750 YBP) turned out to be distant relatives of modern Mongolian-speaking MF36295 mutation carriers. Closer to modern North Altaians is the oldest sample from the Shamanka II burial site (~7700 YBP). Just like North Altaians, the remaining eight samples were positioned on the N-Y147658 branch dated at ~8000–9000 YBP. The sample from Sicilia was in the same branch N-Y147658(xY147918) as the sample from Hungary. The Asian samples AT614, UAA001, DA220, C1664 and C1668 (see Table 1) shared the branch N-Y147918 with the sample C1650 from Xinjiang, China. The observed hierarchy of ancient mutations suggests that the Y148042 mutation is an SNP marker of modern North Altaians.
Of 10 ancient samples, nine were compared with modern samples representing the Balkan subhaplogroup N-P189.2. The whole genome of the oldest sample from Zhokhov Island in the Arctic (~8000 YBP) was yet unavailable for study. There were two samples (Figure 3) with good coverage: one from Kazakhstan (Botai, ~5100 YBP) and the other from Khakassia (Beltyry, ~4700 YBP). Sequence alignment revealed an ancestral allele at position P189.2 in the ancient samples from the Altai territory (Razdum’ye-1) and Khakassia (Beltyry). Therefore, it would be more accurate to name the lineage N-Y6517 after the mutation identified in the majority of its representatives. The earliest to diverge was the branch of one of the most ancient samples (Altai territory, Razdum’ye-1, ~7600 YBP), followed by the branch of the Khakassian sample (Beltyry, ~4700 YBP). The Y6533 mutation was shared by the branches of the ancient Botai sample and all other ancient and modern samples with a TMRCA of 9500 ± 1700 YBP. Among them, there was a sample from Kazakhstan (Sjauke, ~3800 YBP), but its lineage could not be identified due to poor coverage. Individual mutations discovered during the process of alignment in two samples from Kazakhstan (Mereke, 4000–4400 YBP), the Stavropol territory in Russia (Tipki, ~4000 YBP), Moldova (Ciumai, Bronze Age), and Uzbekistan (~2050 YBP) placed these samples in a currently rare branch N-Y111068. We were unable to identify the position of the sample from the Altai territory (Tuzovskie-Bugry-1, ~5400 YBP) in the branches of subhaplogroups N-Y147969 and N-Y6517 due to low genome coverage: perhaps, it constitutes a separate, currently extinct lineage within the haplogroup N-B482.
It is believed that a population that carried different lineages of the Y-chromosome haplogroup N arrived in the Baikal region from Northeast China ~9000 years ago [52]. Not coincidentally, the majority of the oldest N-B482 samples were discovered in the Irkutsk region, Buryatia, and Yakutia (Table 1). In our dataset, South Siberia was represented by ancient samples from the Altai territory (I12695, I13260), Khakassia (I20089, Kh12, Kh13, Kh15), Krasnoyarsk territory (NEO70), Tuva (ARZ-T15), and by samples of modern North Altaians, including Kumandins, Chelkans, and Tubalars. STR haplotypes suggest that two Mongolian samples from the Bronze Age (AT614 and UAA001) are related to the sample from the Okunevo culture Kh12.
Most of the ancient samples representing the Balkan subhaplogroup N-P189.2 (N-Y6517) come from North Kazakhstan. The oldest is the sample BOT15 from the Eneolithic Botai culture. Donors of the samples I11735 and I11737 from the Poltavka culture and the sample NEO900 from the Elunino culture lived in the Bronze Age. Overall, Bronze Age samples belonging to the Balkan subhaplogroup N-P189.2 (N-Y6517) tend to come from more western regions than the samples of the Altaian subhaplogroup N-Y147969: the sample TIP001 from the Lola culture was discovered in the Stavropol territory, Russia, whereas the Bronze Age sample I11926 came from Moldova. Early Iron Age samples of the Altaian subhaplogroup N-Y147969 from Kazakhstan (DA220) and Xinjiang (C1650, C1664, C1668) are geographically close to each other (345 km) and might represent the same population. The sample L8630 was found among the Kushan Empire samples in southern Uzbekistan.
Clearly, the Altaian subhaplogroup N-Y147969 was very widespread in ancient times. Its carriers gradually migrated westward from Lake Baikal and finally arrived in Europe. The male donor of the sample IR1 lived in Pannonia in the Early Iron Age (~2850 YBP) before the arrival of the Scythians. He may have descended from the first nomadic migration led by the Cimmerians from the East of the Eurasian steppe. Two cultural innovations, specifically mare milking (~3350 YBP [53]) and horse riding (~3000 YBP [36]), allowed ancient nomads to travel enormous distances in a short time. The autosomal genomes of Cimmerians and the Karasuk populations of South Siberia are closely related [54], supporting the archaeological hypothesis regarding the Karasuk cultural origin of Cimmerians [55]. Some bearers of the Karasuk culture, in turn, were descendants of the Okunevo culture populations that inhabited the region in earlier times. The donor of the sample I10944/W0461 was a mercenary in the Ancient Greek army [48] and died in 480 BC in a battle with Carthaginians in Sicilia.
Table 1 shows that the subhaplogroup N-Y147969 survived the dramatic reduction in Y-chromosome diversity in the Neolithic time at 8000–4000 YBP [56] and the Late Bronze Age collapse at 3100–3200 YBP. However, most lineages of the Altai subhaplogroup N-Y147969 died out. The remaining relicts include small branches of North Altaians, Mongolian-speaking populations, and some individuals from China (Figure 1). It seems that the Late Bronze Age collapse affected populations within the Balkan subhaplogroup N-P189.2 (N-Y6517) inhabiting the vicinity of copper mines in the South Urals. Only two ancient samples from the Iron Age were found within this subhaplogroup: L8630 from Uzbekistan (Table 1) and a Sarmatian sample from the Lower Volga region [57].
Overall, all modern branches of the haplogroup N-B482 have a very small number of carriers, and most of the branches represented by ancient samples in Table 1 and Figure 3 are now extinct.
4. Discussion
Out of four key factors of microevolution (mutations, migrations, genetic drift and natural selection), migrations and genetic drift may have become the major driving forces in shaping the genetic landscape of the Y-chromosome. Natural selection does not play a significant role in this process because haplogroup-marking mutations are neutral. Besides, the analyzed branches of the haplogroup N-B482 emerged in the past 10,000 years when cultural evolution was far more effective than biological evolution [2]. Mutations led to the emergence of new branches within the haplogroup N-B482 in the past (and still do in the present) (Figure 1 and Figure 2), but did not affect their further trajectory.
Unlike mutations, migrations can not only increase, but also reduce differentiation. The majority of haplogroup N-B482 carriers were nomads from the Bronze Age and Early Iron Age who migrated actively across the Eurasian steppes. The sample IR1 from an Early Iron Age burial in Hungary represents the Pre-Scythian Mezőcsát culture, which was dominated by Cimmerians. In Pre-Scythian times, early nomads from the Karasuk-Arzhan migration wave entered a relatively non-violently foreign Thracian context, creating a Thraco-Cimmerian community [58]. Hypothetically, the arrival of a non-typical Balkan subhaplogroup N-P189.2 (N-Y6517, branch N-FT198615) in the Middle East may have been associated with the military campaigns of the Cimmerians or Scythians in Western Asia during the 8th–6th centuries BC, as described by Herodotus [59].
However, the main factor that reduces diversity among Y-chromosome lineages in small populations is genetic drift. The systematic extinction of individual haplogroup N-B482 lineages is a result of random genetic drift. The bottleneck effect is a form of genetic drift characterized by a dramatic reduction in the population’s size further followed by its growth. In the past, the bottleneck effect could have affected either an entire population (through natural disasters, famine, epidemics, etc.) or primarily its male members (through wars, sex imbalance, etc.) [60,61]. The extinction of some Y-chromosome lineages may have been caused by economic factors that affected population growth rates, such as unequal access to resources. If such factors had a lasting effect, they may have caused the extinction of those Y-chromosome lineages that were less competitive [62]. However, the key factor in eliminating Y-chromosome lineages which exerts its effect even when the analyzed populations are initially equal in size is a continuous universal extinction of male lineages due to the stochastic character of female and male births with a probability close to 0.5. This process was first described by Galton and Watson (see Appendix A).
However, the relict branch of the North Altaians persists to the present day, although the majority of the ancient N-Y147969 subhaplogroup branches have died out. The Altaian subhaplogroup N-Y147969 has survived, while many branches of other haplogroups have become extinct due to the random nature of the universal extinction of paternal lineages. The ancestors of North Altaians within this lineage were reproductively fortunate: they produced more boys than girls, unlike their competitors from other lineages. This saved the relict branch of the N-Y147969 subhaplogroup from extinction. The South Slavic branch within subhaplogroup N-Y6516 is a similar case of accidental reproductive success. Although it is only 750 years old [3], it is one of the most frequent branches within N-B482, reaching a frequency of 3–4% among Serbs (120,000–160,000 male individuals).
5. Conclusions
We have conducted a comprehensive phylogenetic analysis of our own collection and literature data on Y-chromosome lineages of ancient and modern haplogroup N-B482 carriers. This haplogroup was widespread in the Neolithic, Bronze, and Iron Ages, occurring from Baikal to Hungary and from the Arctic to Uzbekistan, but today the number of its carriers is vanishingly small. The Altaian subhaplogroup N-Y147969 now mainly occurs in North Altaians (Kumandins, Chelkans, and Tubalars) and in Mongolian and Chinese populations. Based on whole-genome sequencing data from three N-Y147969 samples (Altai Tubalar, Kalmyk, and Khalkha-Mongolian), we were able to describe a previously unknown phylogenetic structure of the North Altaian (N-Y148042) and Mongolian (N-MF36295) branches within the Altaian subhaplogroup N-Y147969. The rare Chinese lineage is probably derived from N-Y147658, which also includes North Altaians. Further research is needed to establish its precise position in the phylogenetic tree. So far, we know that the Balkan subhaplogroup N-P189.2 (N-Y6517) occurs farther to the west in Europe (mostly in the Balkans) and in the Middle East.
Factors of cultural evolution, such as the Neolithic Revolution or the Bronze Age demographic collapse, do not explain the extinction of N-B482 branches. We claim that the main cause of their elimination is genetic drift, a universal mechanism for the elimination of paternal lineages associated with the stochasticity of female or male births with a probability close to 0.5. The Galton-Watson theory of branching processes demonstrates that this stochastic competition leads to a high extinction probability for Y-chromosome lineages over time.
Supplementary Materials
The following supporting information can be found at: https://www.sciepublish.com/article/pii/730, Table S1: The subhaplogroup N-Y147969 Y-SNP list; Table S2: The haplogroup N-B482 modern samples.
Appendix A
Extinction of Genealogies in the Theory of Galton-Watson Branching Processes
The number of modern descendants of those men who lived in the past can vary significantly depending on a multitude of factors. An attempt to explain the extinction of British aristocratic family names by a stochastic branching process was made by Galton and Watson in the 19th century. The Galton-Watson branching process makes allowance for a variance in the number of male offspring, including the absence thereof, produced by different men. Although the model cannot cover the entire diversity of causes underlying the extinction of male lineages, its simplicity illustrates the universal, overarching character of stochastic elimination of paternal lineages. The Galton-Watson theory of branching processes is outlined in [63,64].
The extinction probability of male lineages depends on the probability distribution for the number of sons $${p}_{0},{p}_{1},{p}_{2},{p}_{3},\dots$$, where $${p}_{0}$$ is the probability of not having sons, $${p}_{1}$$ is the probability of having one son, $${p}_{2}$$ is the probability of having 2 sons, etc. The distribution defines 2 important parameters: the average number of sons $$A$$ and dispersion $${\sigma }^{2}$$.
Alfred Lotka [65] demonstrated that the following distribution well describes the American statistics on male offsprings:
|
|
If we ignore the first member of the distribution $${p}_{0}$$, then this distribution will have the shape of a geometric distribution. For a purely geometric distribution, the first member in the example would equal 1 − 0.5893 = 0.4107, which is slightly lower than the probability suggested by Lotka.
The probability of a stochastic number of offspring from each progenitor for a geometric distribution can be described by the formula:
| , , |
where is the probability of not having sons, k is the number of sons. According to the properties of a geometric distribution, the average number of sons A equals and dispersion .
We are looking at cases in which the number of paternal descendants tends to grow in subsequent generations, i.e., $$A>1$$. In theory, this process is referred to as a supercritical branching process. Generally speaking, the properties of a supercritical branching process depend on the age T of the analyzed lineage. In practice, in most cases, it is possible to use the approximation T→∞ at which large fluctuations characteristic of the lineage’s early generations no longer exert their effects. Approximation to an infinite number of generations applies at $$\left(A-1\right)T \gg 1$$. If this condition is satisfied for the geometric distribution of the number of sons, the extinction probability E is:
| . |
To check the validity of the formula, we simulated the development of genealogies over time using the Monte-Carlo method, assuming that an average number of sons per man is 1.094899. This value was determined by the average number of men in the final population, which equaled 100,000 after $$T=100$$ generations for surviving genealogies. When the number of surviving genealogies reached 10,000, the modeling was terminated. We found that 104,941 genealogies had not survived to the age of 100 generations. The probability of genealogy extinction was estimated at 104,941/(104,941 + 10,000) = 0.9130. The theoretical value 1/1.094899 = 0.9133 was in perfect agreement with the results of computer modeling.
The growth rate of a Neolithic population was about 0.1% a year [66], i.e., A = 1.03, and the average generation time was 30 years. According to the branching process theory, 97% of the Neolithic Y-chromosome lineages (1/1.03 = 0.97) became extinct at this growth rate. But even in a rapidly growing population that doubles with every generation (A = 2), 50% of Y-chromosome lineages become eliminated over time. Therefore, the higher the average number of sons per man, the lower is the extinction probability for the Y-chromosome lineage. At the modern growth rate of the world’s population, which is 0.9% a year (А = 1.3), the extinction probability for its Y-chromosome lineages is 77%.
The universal law of extinction of paternal lineages has its consequences. For example, old branches of the Y-chromosome tree usually split into only two new branches. If the number of male offspring follows a geometric distribution in the Galton-Watson branching process, then the probability of survival of three branches is very low and equals (1 − 1/А) of the probability of survival for two lineages. Another consequence of the extinction law is that the overwhelming majority of ancient men whose aDNA we study today are not direct ancestors of modern men.
Acknowledgments
The authors thank all the sample donors and the Biobank of Northern Eurasia for the access to DNA collections. The authors are grateful to Kirill Gaiman for cooperation and providing data on his own Y-chromosome.
Author Contributions
Conceptualization, D.A. and E.B.; Methodology, D.A.; Software, I.E.; Validation, E.B. and M.Z.; Formal Analysis, D.A. and I.E.; Investigation, D.A. and G.P.; Resources, G.P., M.B., A.A. and R.B.; Data Curation, E.B. and G.P.; Writing—Original Draft Preparation, D.A.; Writing—Review & Editing, D.A., E.B. and M.Z.; Visualization, D.A. and E.B.; Supervision, E.B.; Project Administration, E.B.; Funding Acquisition, E.B. All authors have read and approved the final manuscript.
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Bochkov Research Centre for Medical Genetics (protocol No. 1 of 29 June 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data used in the paper are provided in Supplementary Materials
Funding
This research was funded by the state assignment of the Ministry of Science and Higher Education of the Russian Federation to the Bochkov Research Centre for Medical Genetics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ilumäe AM, Reidla M, Chukhryaeva M, Järve M, Post H, Karmin M, et al. Human Y chromosome haplogroup N: A non-trivial time-resolved phylogeography that cuts across language families. Am. J. Hum. Genet. 2016, 99, 163–173. doi:10.1016/j.ajhg.2016.05.025. [Google Scholar]
- Chiaroni J, Underhill PA, Cavalli-Sforza LL. Y chromosome diversity, human expansion, drift, and cultural evolution. Proc. Natl Acad. Sci. USA 2009, 106, 20174–20179. doi:10.1073/pnas.0910803106. [Google Scholar]
- YFull YTree v13.05.00. 2025. Available online: https://www.yfull.com/tree/ (accessed on 9 August 2025). [Google Scholar]
- Kharkov VN, Stepanov VA, Medvedeva OF, Spiridonova MG, Voevoda MI, Tadinova VN, et al. Gene Pool Differences between Northern and Southern Altaians Inferred from the Data on Y-Chromosomal Haplogroups. Russ. J. Genet. 2007, 43, 551–562. doi:10.1134/S1022795407050110. [Google Scholar]
- Balaganskaya OA, Lavryashina MB, Kuznetchova MA, Romanov AG, Dibirova KhD, Frolova SA, et al. Gene pool of the Altai ethnic groups (from Russia, Kazakhstan, and Mongolia) analyzed by the Y chromosomal markers. Mosc. Univ. Anthropol. Bull. 2011, 2, 25–36. (In Russian) [Google Scholar]
- Dulik MC, Zhadanov SI, Osipova LP, Askapuli A, Gau L, Gokcumen O, et al. Mitochondrial DNA and Y chromosome variation provides evidence for a recent common ancestry between Native Americans and Indigenous Altaians. Am. J. Hum. Genet. 2012, 90, 229–246. doi:10.1371/journal.pone.0017548. [Google Scholar]
- Damgaard PDB, Martiniano R, Kamm J, Moreno-Mayar JV, Kroonen G, Peyrot M, et al. The first horse herders and the impact of early Bronze Age steppe expansions into Asia. Science 2018, 360, eaar7711. doi:10.1126/science.aar7711. [Google Scholar]
- King T, Jobling M. Founders, Drift, and Infidelity: The Relationship between Y Chromosome Diversity and Patrilineal Surnames. Mol. Biol. Evol. 2009, 26, 1093–1102. doi:10.1093/molbev/msp022. [Google Scholar]
- Underhill PA, Passarino G, Lin AA, Shen P, Mirazón Lahr M, Foley RA, et al. The phylogeography of Y chromosome binary haplotypes and the origins of modern human populations. Ann. Hum. Genet. 2001, 65 Pt 1, 43–62. doi:10.1046/j.1469-1809.2001.6510043.x. [Google Scholar]
- Balanovska EV, Zhabagin MK, Agdzhoyan AT, Chukhryaeva MI, Markina NV, Balaganskaya OA, et al. Population Biobanks: Organizational Models and Prospects of Application in Gene Geography and Personalized Medicine. Russ. J. Genet. 2016, 52, 1227–1243. doi:10.1134/S1022795416120024. [Google Scholar]
- Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. doi:10.1093/oxfordjournals.molbev.a026036. [Google Scholar]
- Zhivotovsky LA, Underhill PA, Cinnioğlu C, Kayser M, Morar B, Kivisild T, et al. The effective mutation rate at Y chromosome short tandem repeats, with application to human population-divergence time. Am. J. Hum. Genet. 2004, 1, 50–61. doi:10.1086/380911. [Google Scholar]
- Willuweit S, Roewer L. The new Y Chromosome Haplotype Reference Database. Forensic Sci. Int. Genet. 2015, 15, 43–48. doi:10.1016/j.fsigen.2014.11.024. [Google Scholar]
- Fenner J. Cross-cultural estimation of the human generation interval for use in genetics-based population divergence studies. Am. J. Phys. Anthropol. 2005, 128, 415–423. doi:10.1002/ajpa.20188. [Google Scholar]
- Adamov D, Guryanov V, Karzhavin S, Tagankin V, Urasin V. Defining a new rate constant for Y-chromosome SNPs based on full sequencing data. Russ. J. Genet. Geneal. 2015, 1, 3–36. [Google Scholar]
- Balanovsky O. Toward a consensus on SNP and STR mutation rates on the human Y-chromosome. Hum. Genet. 2017, 136, 575–590. doi:10.1007/s00439-017-1805-8. [Google Scholar]
- Marchi N, Hegay T, Mennecier P, Georges M, Laurent R, Whitten M, et al. Sex-specific genetic diversity is shaped by cultural factors in Inner Asian human populations. Am. J. Phys. Anthropol. 2017, 162, 627–640. doi:10.1002/ajpa.23151. [Google Scholar]
- Yekeev NV. Altai: History and Culture (Selected Works); Research Institute of Altaic Studies named after S.S. Surazakov; Gorno-Altaisk Printing House: Gorno-Altaisk, Russia, 2015; pp. 175–192. (In Russian) [Google Scholar]
- Slavnin VD, Sherstova LI. Culture and Traditions of the Indigenous Peoples of Northern Altai; Malinov AV, Ed.; Publishing House of St. Petersburg State University: St. Petersburg, Russia, 2008; pp. 55–93. (In Russian) [Google Scholar]
- Potapov LP. Ethnic Composition and Origin of the Altaians; Historical and ethnographic essay; Okladnikov AP, Ed.; Nauka: Leningrad, Russia, 1969; pp. 147–196. (In Russian) [Google Scholar]
- Kačar T, Stamenković G, Blagojević J, Krtinić J, Mijović D, Marjanović D. Y chromosome genetic data defined by 23 short tandem repeats in a Serbian population on the Balkan Peninsula. Ann. Hum. Biol. 2019, 46, 77–83. doi:10.1080/03014460.2019.1584242. [Google Scholar]
- Zgonjanin D, Alghafri R, Antov M, Stojiljković G, Petković S, Vuković R, et al. Genetic characterization of 27 Y-STR loci with the Yfiler® Plus kit in the population of Serbia. Forensic Sci. Int. Genet. 2017, 31, e48–e49. doi:10.1016/j.fsigen.2017.07.013. [Google Scholar]
- Mihajlovic M, Tanasic V, Keckarevic Markovic M, Kecmanovic M, Keckarevic D. Distribution of Y-chromosome haplogroups in Serbian population groups originating from historically and geographically significant distinct parts of the Balkan Peninsula. Forensic Sci. Int. Genet. 2022, 61, 102767. doi:10.1016/j.fsigen.2022.102767. [Google Scholar]
- Mršić G, Gršković B, Vrdoljak A, Popović M, Valpotić I, Anđelinović S, et al. Croatian national reference Y-STR haplotype database. Mol. Biol. Rep. 2012, 39, 7727–7741. doi:10.1007/s11033-012-1610-3. [Google Scholar]
- Sterlinko H, Pajnic IZ, Balazic J, Komel R. Human Y-specific STR haplotypes in a Slovenian population sample. Forensic Sci. Int. 2001, 120, 226–228. doi:10.1016/s0379-0738(01)00390-5. [Google Scholar]
- Jordamović B, Kojović T, Dogan S, Bešić L, Salihefendić L, Konjhodžić R, et al. Contemporary population genetics data for 23 Y-STR loci in the general Bosnian-Herzegovinian population. Genet. Appl. 2021, 5, 51–63. doi:10.31383/ga.vol5iss1pp51-63. [Google Scholar]
- Nothnagel M, Fan G, Guo F, He Y, Hou Y, Hu S, et al. Revisiting the male genetic landscape of China: A multi-center study of almost 38,000 Y-STR haplotypes (Retraction of Hum. Genet. 2017, 136, 485–497). Hum. Genet. 2022, 141, 175–176. doi:10.1007/s00439-021-02413-w. [Google Scholar]
- Wang C-Z, Su M-J, Li Y, Chen L, Jin X, Wen S-Q, et al. Genetic polymorphisms of 27 Yfiler® Plus loci in the Daur and Mongolian ethnic minorities from Hulunbuir of Inner Mongolia Autonomous Region, China. Forensic Sci. Int. Genet. 2019, 40, e252–e255. doi:10.1016/j.fsigen.2019.02.003. [Google Scholar]
- Malyarchuk B, Derenko M, Denisova G, Khoyt S, Woźniak M, Grzybowski T, et al. Y-chromosome diversity in the Kalmyks at the ethnical and tribal levels. J. Hum. Genet. 2013, 58, 804–811. doi:10.1038/jhg.2013.108. [Google Scholar]
- Fu X, Fu Y, Liu Y, Guo J, Liu Y, Guo Y, et al. Genetic polymorphisms of 26 Y-STR loci in the Mongolian minority from Horqin district, China. Int. J. Legal Med. 2016, 130, 941–946. doi:10.1007/s00414-016-1387-3. [Google Scholar]
- Wang M, He G, Zou X, Liu J, Ye Z, Ming T, et al. Genetic insights into the paternal admixture history of Chinese Mongolians via high-resolution customized Y-SNP SNaPshot panels. Forensic Sci. Int. Genet. 2021, 54, 102565. doi:10.1016/j.fsigen.2021.102565. [Google Scholar]
- Wu R, Li R, Wang N, Peng D, Li H, Zhang Y, et al. Genetic polymorphism and population structure of Torghut Mongols and comparison with a Mongolian population 3000 km away. Forensic Sci. Int. Genet. 2019, 42, 235–243. doi:10.1016/j.fsigen.2019.07.017. [Google Scholar]
- Roewer L, Willuweit S, Krüger C, Nagy M, Rychkov S, Morozowa I, et al. Analysis of Y chromosome STR haplotypes in the European part of Russia reveals high diversities but non-significant genetic distances between populations. Int. J. Legal Med. 2008, 122, 219–223. doi:10.1007/s00414-007-0222-2. [Google Scholar]
- Sutherland AMS. The Palaeogenomics of Arctic and Sub-Arctic Peoples: A Study of Population Genetics, Adaptations, and Pathogen Incidence. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2023. [Google Scholar]
- Zeng TC, Vyazov LA, Kim A, Flegontov P, Sirak K, Maier R, et al. Postglacial genomes from foragers across Northern Eurasia reveal prehistoric mobility associated with the spread of the Uralic and Yeniseian languages. bioRxiv 2023, preprint. doi:10.1101/2023.10.01.560332. [Google Scholar]
- Jeong C, Wang K, Wilkin S, Taylor WTT, Miller BK, Bemmann JH, et al. A Dynamic 6000-Year Genetic History of Eurasia’s Eastern Steppe. Cell 2020, 183, 890–904.e29. doi:10.1016/j.cell.2020.10.015. [Google Scholar]
- Lazaridis I, Patterson N, Anthony D, Vyazov L, Fournier R, Ringbauer H, et al. The Genetic Origin of the Indo-Europeans. Nature 2025, 639, 132–142. doi:10.1038/s41586-024-08531-5. [Google Scholar]
- Hollard C. Peopling of Southern Siberia and Altai during the Bronze Age: Contribution of Paleogenetics. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2014. (In French) [Google Scholar]
- Hollard C, Zvénigorosky V, Kovalev A, Kiryushin Y, Tishkin A, Lazaretov I, et al. New genetic evidence of affinities and discontinuities between bronze age Siberian populations. Am. J. Phys. Anthropol. 2018, 167, 97–107. doi:10.1002/ajpa.23607. [Google Scholar]
- Narasimhan VM, Patterson N, Moorjani P, Rohland N, Bernardos R, Mallick S, et al. The formation of human populations in South and Central Asia. Science 2019, 365, eaat7487. doi:10.1126/science.aat7487. [Google Scholar]
- Ghalichi A, Reinhold S, Rohrlach AB, Kalmykov AA, Childebayeva A, Yu H, et al. The rise and transformation of Bronze Age pastoralists in the Caucasus. Nature 2024, 635, 917–925. doi:10.1038/s41586-024-08113-5. [Google Scholar]
- Allentoft ME, Sikora M, Refoyo-Martínez A, Irving-Pease EK, Fischer A, Barrie W, et al. Population genomics of post-glacial western Eurasia. Nature 2024, 625, 301–311. doi:10.1038/s41586-023-06865-0. [Google Scholar]
- Wang C-C, Yeh H-Y, Popov AN, Zhang H-Q, Matsumura H, Sirak K, et al. Genomic insights into the formation of human populations in East Asia. Nature 2021, 591, 413–419. doi:10.1038/s41586-021-03336-2. [Google Scholar]
- Harney É, Cheronet O, Fernandes DM, Sirak K, Mah M, Bernardos R, et al. A minimally destructive protocol for DNA extraction from ancient teeth. Genome Res. 2021, 31, 472–483. doi:10.1101/gr.267534.120. [Google Scholar]
- Gamba C, Jones ER, Teasdale MD, McLaughlin RL, Gonzalez-Fortes G, Mattiangeli V, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat. Commun. 2014, 5, 5257. doi:10.1038/ncomms6257. [Google Scholar]
- Allentoft ME, Sikora M, Sjögren K-G, Rasmussen S, Rasmussen M, Stenderup J, et al. Population genomics of Bronze Age Eurasia. Nature 2015, 522, 167–172. doi:10.1038/nature14507. [Google Scholar]
- Mary L, Zvénigorosky V, Kovalev A, Gonzalez A, Fausser J-L, Jagorel F, et al. Genetic kinship and admixture in Iron Age Scytho-Siberians. Hum. Genet. 2019, 138, 411–423. doi:10.1007/s00439-019-02002-y. [Google Scholar]
- Reitsema LJ, Mittnik A, Kyle B, Catalano G, Fabbri PF, Kazmi ACS, et al. The diverse genetic origins of a Classical period Greek army. Proc. Natl Acad. Sci. USA 2022, 119, e2205272119. doi:10.1073/pnas.2205272119. [Google Scholar]
- Kumar V, Wang W, Zhang J, Wang Y, Ruan Q, Yu J, et al. Bronze and Iron Age population movements underlie Xinjiang population history. Science 2022, 376, 62–69. doi:10.1126/science.abk1534. [Google Scholar]
- Damgaard PDB, Marchi N, Rasmussen S, Peyrot M, Renaud G, Korneliussen T, et al. 137 ancient human genomes from across the Eurasian steppes. Nature 2018, 557, 369–374. doi:10.1038/s41586-018-0094-2. [Google Scholar]
- Kumar V, Bennett EA, Zhao D, Liang Y, Tang Y, Ren M, et al. Genetic Continuity of Bronze Age Ancestry with Increased Steppe-Related Ancestry in Late Iron Age Uzbekistan. Mol. Biol. Evol. 2021, 38, 4908–4917. [Google Scholar]
- Kılınç GM, Kashuba N, Koptekin D, Bergfeldt N, Dönertaş HM, Rodríguez-Varela R, et al. Human population dynamics and Yersinia pestis in ancient northeast Asia. Sci. Adv. 2021, 7, eabc4587. doi:10.1093/molbev/msab216. [Google Scholar]
- Miller A, Wilkin S, Hendy J, Turbat T, Batsukh D, Bayarkhuu N, et al. The spread of herds and horses into the Altai: How livestock and dairying drove social complexity in Mongolia. PLoS ONE 2022, 17, e0265775. doi:10.1371/journal.pone.0265775. [Google Scholar]
- Krzewińska M, Kılınç GM, Juras A, Koptekin D, Chyleński M, Nikitin AG, et al. Ancient genomes suggest the eastern Pontic-Caspian steppe as the source of western Iron Age nomads. Sci. Adv. 2018, 4, eaat4457. doi:10.1126/sciadv.aat4457. [Google Scholar]
- Novozhenov VA. Whence the cimmerians came? transcontinental communications of the early nomads in the lights of the origin of the cimmerians. J. His. Arch. Anthropol. Sci. 2018, 3, 10‒23. doi:10.15406/jhaas.2018.03.00058. [Google Scholar]
- Karmin M, Saag L, Vicente M, Wilson Sayres MA, Järve M, Talas UG, et al. A recent bottleneck of Y chromosome diversity coincides with a global change in culture. Genome Res. 2015, 25, 459–466. doi:10.1101/gr.186684.114. [Google Scholar]
- Pilipenko AS, Cherdantsev SV, Trapezov RO, Tomilin MA, Balabanova MA, Pristyazhnyuk MS, et al. On the issue of the Sarmatian population genetic composition in the Lower Volga region (paleogenetic data). Sci. J. Volgogr. State Univ. Hist. Area Stud. Int. Relat. 2020, 25, 17–50. doi:10.15688/jvolsu4.2020.4.2. (In Russian) [Google Scholar]
- Bruyako IV. Cultural Genesis in the Black Sea-Carpathian Region in the Early Iron Age (The 1st Half of the 1st Millennium BC). Ph.D. Thesis, Institute for the History of Material Culture of RAS, St. Petersburg, Russia, 2004. (In Russian) [Google Scholar]
- Herodotus. The Histories Book 4: Melpomene. In The Nine Books of History; Stratanovsky GA, Translator; Nauka: Leningrad, Russia, 1972; pp. 187–238. (In Russian) [Google Scholar]
- Yan S, Wang C-C, Zheng H-X, Wang W, Qin Z-D, Wei L-H, et al. Y chromosomes of 40% Chinese descend from three Neolithic super-grandfathers. PLoS ONE 2014, 9, e105691. doi:10.1371/journal.pone.0105691. [Google Scholar]
- Zeng TC, Aw AJ, Feldman MW. Cultural hitchhiking and competition between patrilineal kin groups explain the post-Neolithic Y-chromosome bottleneck. Nat. Commun. 2018, 9, 2077. doi:10.1038/s41467-018-04375-6. [Google Scholar]
- Guyon L, Guez J, Toupance B, Heyer E, Chaix R. Patrilineal segmentary systems provide a peaceful explanation for the post-Neolithic Y-chromosome bottleneck. Nat. Commun. 2024, 15, 3243. doi:10.1038/s41467-024-47618-5. [Google Scholar]
- Sevastianov BA. Branching Processes; Nauka: Moscow, Russia, 1971; pp. 49–86. (In Russian) [Google Scholar]
- Athreya KB, Ney P. Branching Processes; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1972; pp. 1–62. [Google Scholar]
- Feller W. An Introduction to Probability Theory and Its Applications; John Wiley & Sons, Inc.: New York, NY, USA; London, UK; Sydney, Australia, 1961; Volume 1, pp. 296–297. [Google Scholar]
- Carneiro RL, Hilse DF. On determining the probable rate of population growth during the neolithic. Am. Anthropol. 1966, 68, 177–181. [Google Scholar]