Found 2 results
Open Access
Article
27 November 2025Lyz1-Expressing Alveolar Type II Cells Contribute to Lung Regeneration
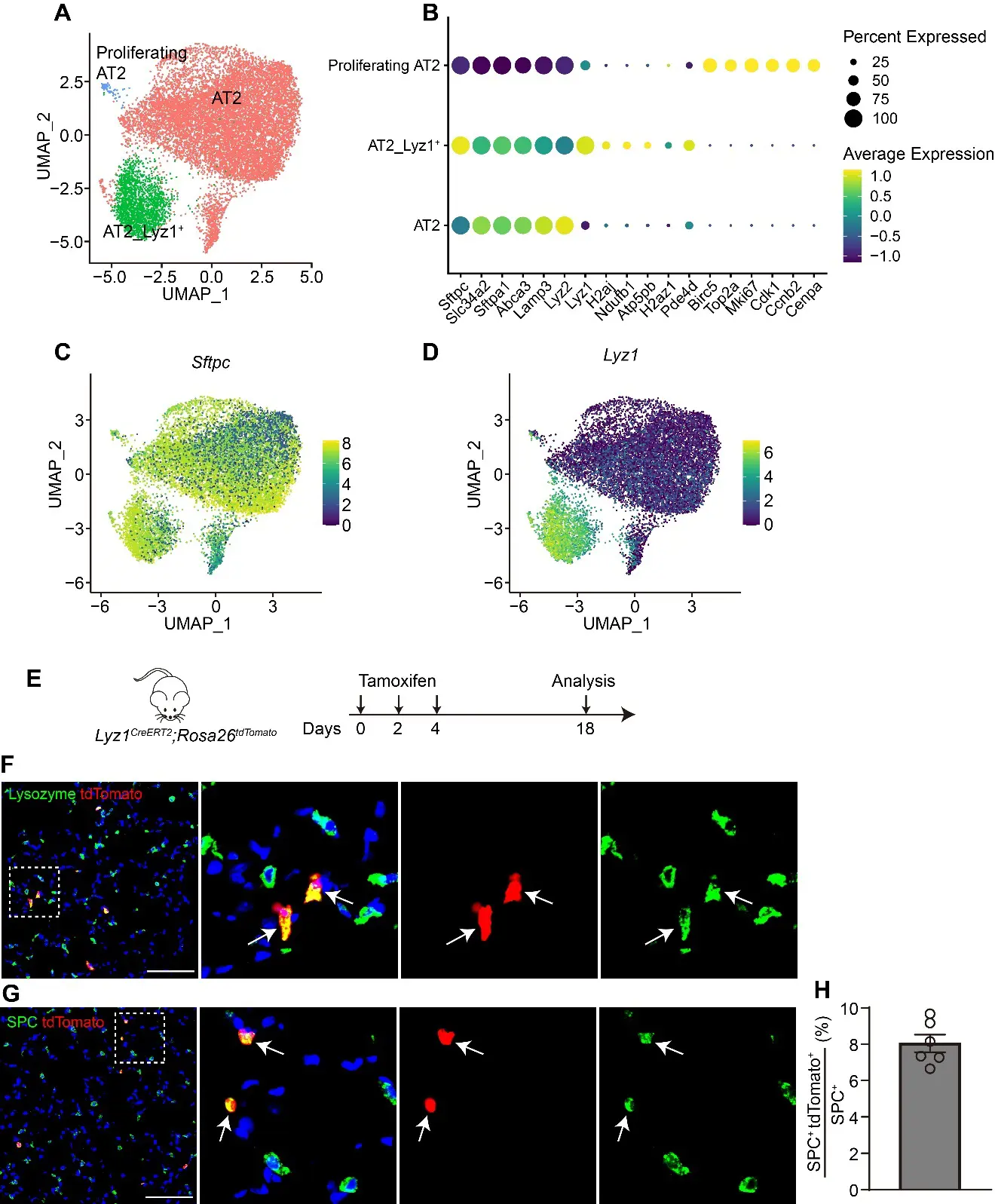
The alveolar units, composed of alveolar epithelial type II cells (AT2) and type I cells (AT1), are essential for efficient gas exchange. While AT2 cells are known to play critical roles in alveolar homeostasis and regeneration, the contribution of heterogeneous AT2 cells to lung repair remains poorly understood. Here, we identified a distinct AT2 subpopulation that exclusively expressed Lysozyme 1 (Lyz1) through single-cell RNA sequencing (scRNA-seq) analyses. Cell fate mapping revealed that the Lyz1CreERT2 mouse strain specifically labeled Lyz1-expressing AT2 cells in vivo at homeostasis. Following lung injury, Lyz1+ AT2 cells expanded and contributed to alveolar regeneration by generating both self-renewing AT2 cells and differentiating AT1 cells. We further observed the emergence of de novo Lyz1-expressing cells in the airways after lung injury. Additionally, Lyz1+ AT2 cells displayed significantly enhanced proliferative capacity compared with general bulk AT2 cells in 3D organoid cultures. These findings define Lyz1+ AT2 cells as a previously unrecognized progenitor population, expanding the paradigm of alveolar regeneration and providing insight into how epithelial diversity supports lung regeneration.
Open Access
Article
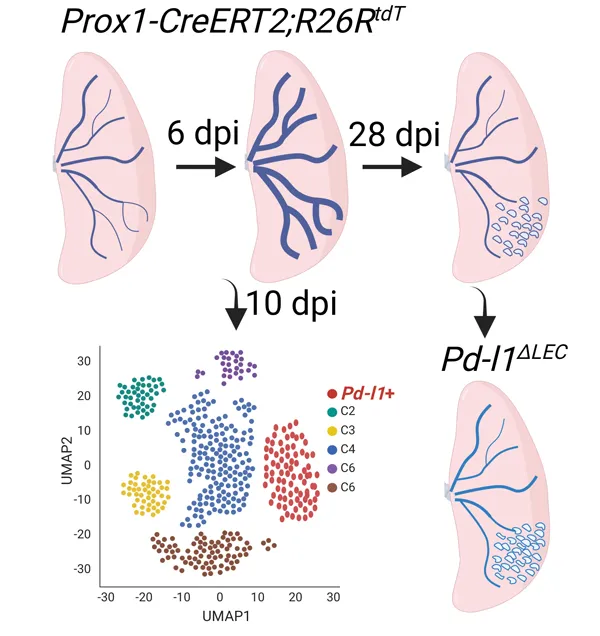
19 February 2024Single Cell Analysis of Lung Lymphatic Endothelial Cells and Lymphatic Responses during Influenza Infection
Tissue lymphatic vessels network plays critical roles in immune surveillance and tissue homeostasis in response to pathogen invasion, but how lymphatic system per se is remolded during infection is less understood. Here, we observed that influenza infection induces a significant increase of lymphatic vessel numbers in the lung, accompanied with extensive proliferation of lymphatic endothelial cells (LECs). Single-cell RNA sequencing illustrated the heterogeneity of LECs, identifying a novel PD-L1+ subpopulation that is present during viral infection but not at steady state. Specific deletion of Pd-l1 in LECs elevated the expansion of lymphatic vessel numbers during viral infection. Together these findings elucidate a dramatic expansion of lung lymphatic network in response to viral infection, and reveal a PD-L1+ LEC subpopulation that potentially modulates lymphatic vessel remolding.