Found 7 results
Open Access
Article
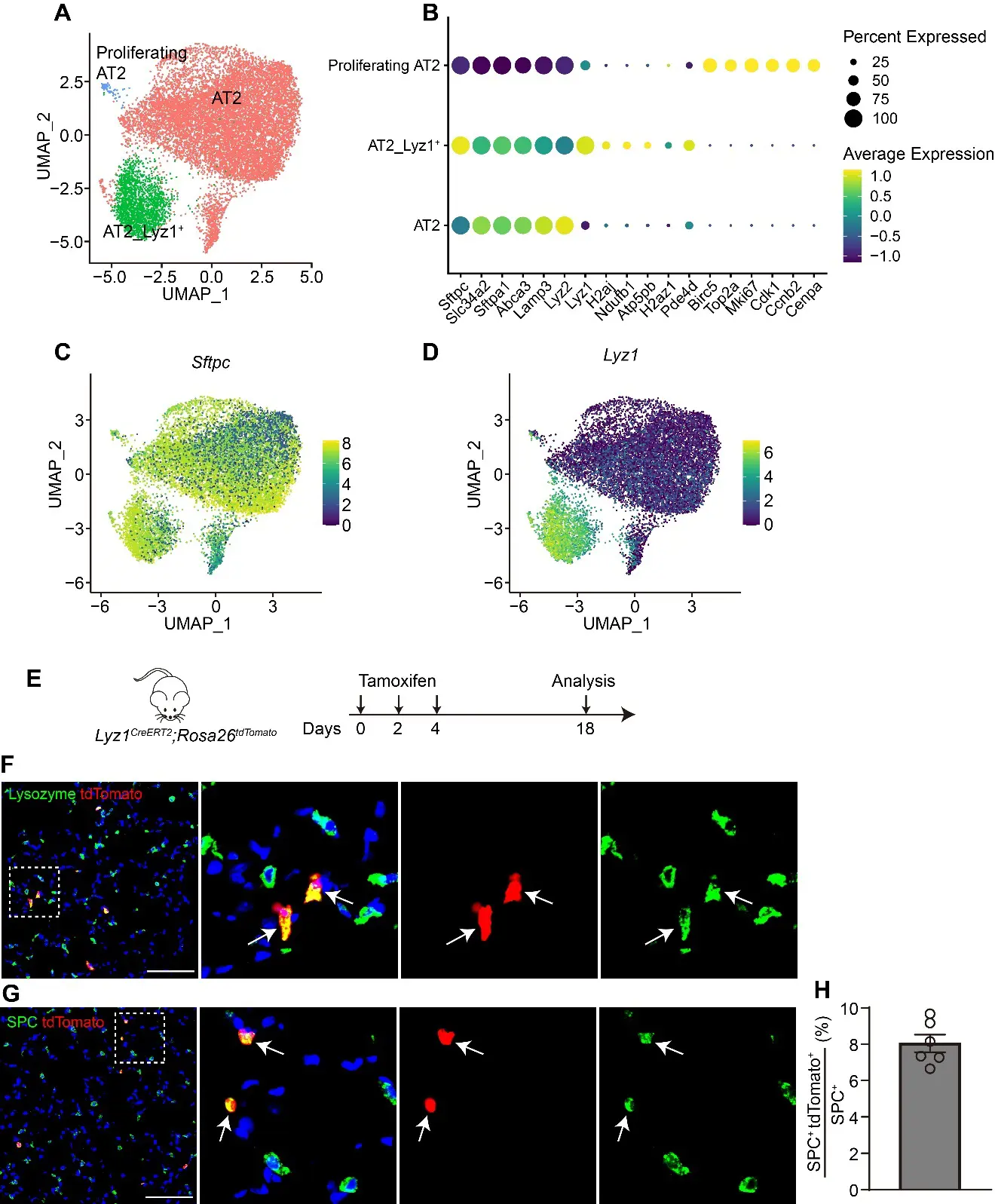
27 November 2025Lyz1-Expressing Alveolar Type II Cells Contribute to Lung Regeneration
The alveolar units, composed of alveolar epithelial type II cells (AT2) and type I cells (AT1), are essential for efficient gas exchange. While AT2 cells are known to play critical roles in alveolar homeostasis and regeneration, the contribution of heterogeneous AT2 cells to lung repair remains poorly understood. Here, we identified a distinct AT2 subpopulation that exclusively expressed Lysozyme 1 (Lyz1) through single-cell RNA sequencing (scRNA-seq) analyses. Cell fate mapping revealed that the Lyz1CreERT2 mouse strain specifically labeled Lyz1-expressing AT2 cells in vivo at homeostasis. Following lung injury, Lyz1+ AT2 cells expanded and contributed to alveolar regeneration by generating both self-renewing AT2 cells and differentiating AT1 cells. We further observed the emergence of de novo Lyz1-expressing cells in the airways after lung injury. Additionally, Lyz1+ AT2 cells displayed significantly enhanced proliferative capacity compared with general bulk AT2 cells in 3D organoid cultures. These findings define Lyz1+ AT2 cells as a previously unrecognized progenitor population, expanding the paradigm of alveolar regeneration and providing insight into how epithelial diversity supports lung regeneration.
Open Access
Review
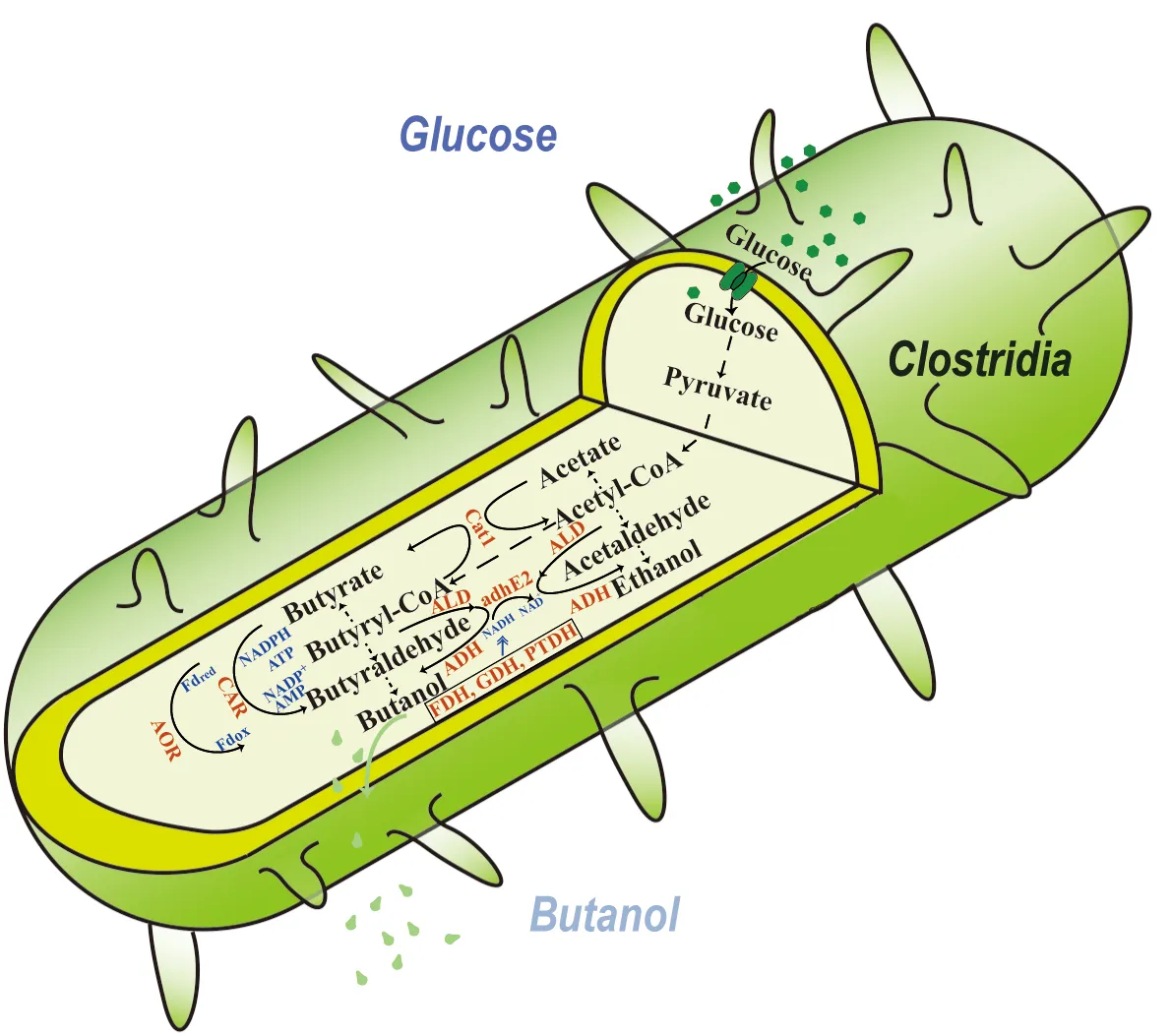
24 March 2025Recent Advances and Challenges in Engineering Metabolic Pathways and Cofactor Regeneration for Enhanced n-Butanol Biosynthesis
The biological production of n-butanol has seen renewed interest due to the need for the production of sustainable aviation fuel, for which n-butanol serves as a direct precursor. However, biological production of this alcohol is still limited by the fermentation’s low titers and low yields. Many approaches have been taken to increase n-butanol production, such as using alternative host organisms, utilizing heterologous enzymes for acid reduction and cofactor regeneration, and protein engineering of critical enzymes in the n-butanol production metabolic pathway. This review highlights key achievements made in each of these areas and shows the potential for these approaches in increasing n-butanol production. The review closes by pinpointing the challenges and limitations in these approaches and recommends that the ultimate approach to n-butanol production should inevitably utilize noncanonical redox cofactors to drive metabolic flux for butanol biosynthesis from glucose.
Open Access
Opinion
21 March 2025Classical MHC Class I Molecules as Modifiers of Brain Homeostasis and Neuroregeneration: Unraveling the Riddle?
As mankind breaks the boundaries of potential years to live, the process of aging imposes various cellular challenges, from less capacity of cell repair and damage to impaired protein formation, causing chronic low-level inflammation on tissues including the brain. Persistent chronic neuroinflammation can harm neurons, contributing to the development of neurodegeneration, a pathological process that affects cognitive function and is often reflected by dementia. This opinion article tries to recapitulate the influence that major histocompatibility class I (MHC-I) molecules have on brain homeostasis and how abnormalities in their expression can lead to cognitive deterioration. Studies carried out during recent years not only demonstrated that neurons and other central nervous system (CNS) cells express MHC-I molecules, but also that these molecules play essential roles in the establishment, function, and modeling of synapses in the CNS during the embryonic period, at birth and during adulthood, namely during inflammatory conditions. The accumulated body of evidence suggests that MHC-I molecules and the signaling pathways they regulate could provide clues on some of the molecular and cellular mechanisms regulating brain homeostasis and neuroregeneration in health and disease, thus becoming potential biomarkers of cognitive decline and targets for innovative immunotherapies.
Open Access
Article
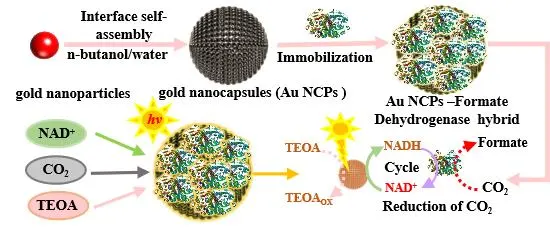
27 November 2024Photocatalytic CO2 Fixation into Formate under Visible Light by the Photo-Enzyme Hybrid of Gold Nanocapsules and Formate Dehydrogenase
The photo-enzyme hybrid system presents a promising approach for the selective conversion of CO2 into valuable chemicals. However, its high dependence on the expensive coenzyme nicotinamide adenine dinucleotide reduced form (NADH), coupled with the need for external electron mediators and highly active photocatalysts, limits its widespread application. Here, we developed a gold nanocapsule—formate dehydrogenase (FDH) hybrid system for in situ NADH regeneration to facilitate the light-driven conversion of CO2 to formate. The results demonstrated that gold nanocapsules (Au NCPs), in conjunction with triethanolamine (TEOA), protected 83.67% of NADH from photodegradation. Under light-driven conditions with TEOA as the electron donor and without external electron mediators, the Au NCPs catalyzed in situ NADH regeneration, achieving a regeneration yield of 22.65%. This process aided FDH in reducing CO2 to formate, resulting in a production rate of 67.40 µmol/L/h. This research provides valuable insights for developing photo-enzyme hybrid systems that efficiently convert CO2 without the need for external electron mediators.
Open Access
Commentary
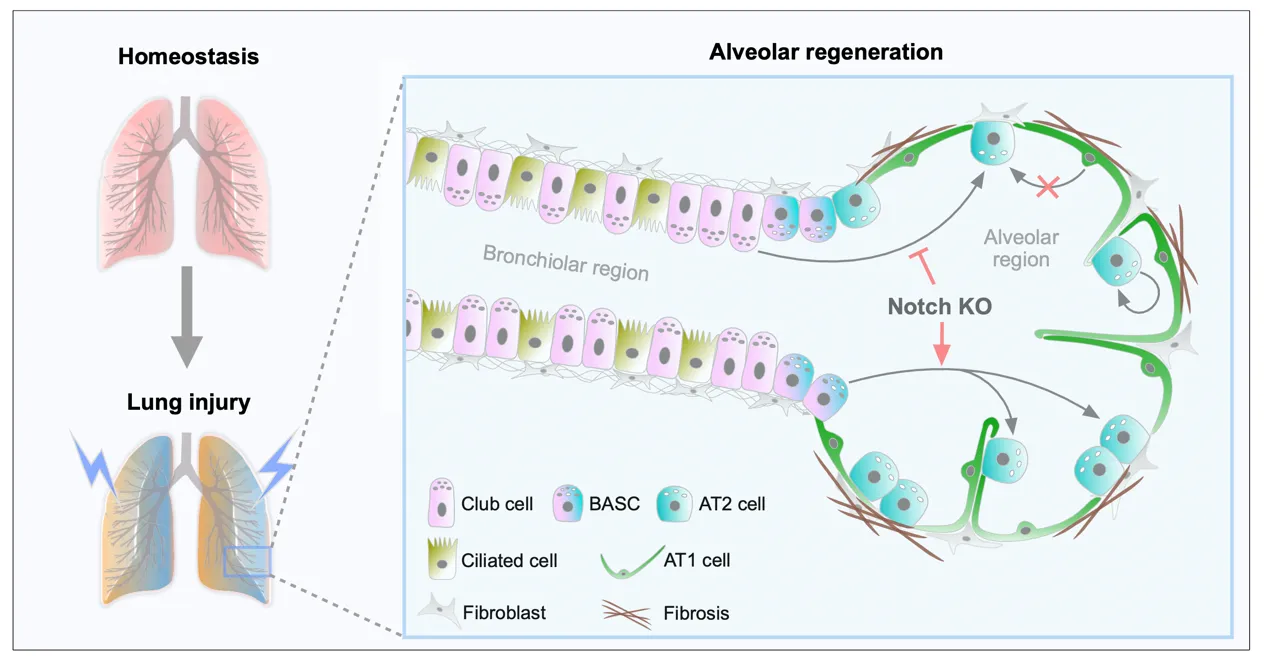
12 June 2024Dual Genetic Tracing Reveals the Origin of Alveolar Stem Cells after Lung Injury
As alveolar epithelial stem cells, alveolar type II (AT2) cells play a pivotal role in sustaining alveolar homeostasis and facilitating repair processes. However, the sources of AT2 cell regeneration have remained contentious due to the non-specific labeling limitations of traditional single recombinase-based lineage tracing techniques. To address this issue, we employed dual recombination systems to develop more precise lineage tracing methodologies, effectively bypassing the shortcomings of conventional approaches and enabling specific labeling of lung epithelial cells. Our findings demonstrate that, following lung injury, regenerated AT2 cells do not originate from alveolar type I (AT1) cells, but instead derive from bronchiolar club cells and bronchioalveolar stem cells (BASCs), alongside the self-renewal of resident AT2 cells. Furthermore, we discovered that the transition of club cells and BASCs into AT2 cells is distinctly modulated by the Notch signaling pathway. This study not only provides novel insights into lung regeneration, but the innovative lineage tracing technology developed herein also holds promise as a technical support for research in diverse fields.
Open Access
Perspective
04 March 2024Trees—Protectors against a Changing Climate
There are estimated to be about 3 trillion trees on Earth, or about half the number that existed before the dawn of human civilization. Trees are vital to at least four major biogeochemical cycles, namely, the carbon, water, nitrogen and oxygen cycles. In addition to absorbing carbon, and releasing oxygen through photosynthesis, trees are critical for maintaining biodiversity, providing habitat for 80% of land based wildlife, feeding the soil, generating clouds and increasing albedo (thus causing global cooling), influencing rainfall and weather patterns. The loss of trees, therefore, weakens our chances of reaching climate and biodiversity targets, and so proforestation and other practices to stringently preserve the functionality of and holistically restore forest ecosystems, must be adopted as a matter of urgency, paying due attention to soil, and species diversity including mycorrhizae; not being limited to insouciant “tree planting” solutions. Indeed, due to the tardiness of our actions to repair the Earth and its climate, severe restrictions to the cutting of mature trees must actually be enabled globally. However, this alone is not enough, and must be integrated with other forms of land, wetland, grassland and agricultural protection and restoration. Such Nature Based Solutions could provide over one-third of the climate mitigation needed by 2030 to keep within the 2 °C global heating limit. Nonetheless, it is also critical to curb greenhouse gas emissions at source, not only by implementing low-carbon, renewable energy, but also energy demand reduction strategies, such as insulating buildings, societal relocalisation, and local food growing.

Open Access
Article
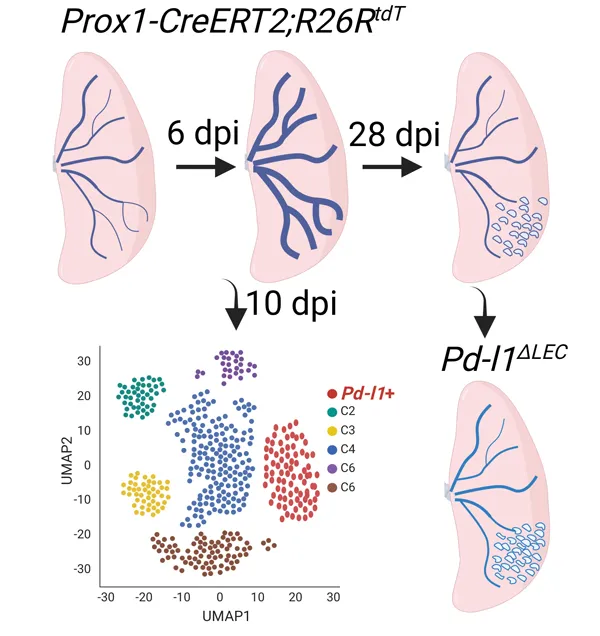
19 February 2024Single Cell Analysis of Lung Lymphatic Endothelial Cells and Lymphatic Responses during Influenza Infection
Tissue lymphatic vessels network plays critical roles in immune surveillance and tissue homeostasis in response to pathogen invasion, but how lymphatic system per se is remolded during infection is less understood. Here, we observed that influenza infection induces a significant increase of lymphatic vessel numbers in the lung, accompanied with extensive proliferation of lymphatic endothelial cells (LECs). Single-cell RNA sequencing illustrated the heterogeneity of LECs, identifying a novel PD-L1+ subpopulation that is present during viral infection but not at steady state. Specific deletion of Pd-l1 in LECs elevated the expansion of lymphatic vessel numbers during viral infection. Together these findings elucidate a dramatic expansion of lung lymphatic network in response to viral infection, and reveal a PD-L1+ LEC subpopulation that potentially modulates lymphatic vessel remolding.