Found 2 results
Open Access
Article
11 October 2024Surfactant Protein-C Regulates Alveolar Type 2 Epithelial Cell Lineages via the CD74 Receptor
Deficiency of surfactant protein-C (SPC) increases susceptibility to lung infections and injury, and suppressed expression of SPC has been associated with the severity of acute respiratory distress syndrome (ARDS). Alveolar type 2 epithelial cells (AT2) are critical for maintenance and repair of the lung. However, the role of the SPC in the regulation of AT2 cell lineage and the underlying mechanisms are not completely understood. This study aimed to investigate the mechanisms by which SPC regulates AT2 lineages. Sftpc−/− mice were used to model the SPC deficiency in ARDS patients. We utilized three-dimensional (3D) organoids to compare AT2 lineage characteristics between wild type (WT) and Sftpc−/− mice by analyzing AT2 proliferation, alveolar type 1 cells (AT1) differentiation and CD74 expression, using colony-formation assay, immunofluorescence, flow cytometry, and immunoblots. The results showed that Sftpc−/− mice demonstrated a reduced AT2 cell population. Influenza A virus subtype H1N1 (H1N1) infected Sftpc−/− mice demonstrated reduced AT2 proliferation and AT1 differentiation. Western blot indicated elevated levels of CD74 protein in AT2 cells of Sftpc−/− mice. Colony-forming efficiency was significantly attenuated in AT2 cells isolated from Sftpc−/− mice compared to the WT controls. Podoplanin (PDPN, a marker of AT1 cells) expression and transient cell count significantly increased in Sftpc−/− organoids. Moreover, siRNA-mediated gene silencing of CD74 in AT2 cells significantly increased AT2 proliferation and AT1 differentiation in Sftpc−/− organoids. This study suggests that SPC regulates AT2 lineage in vitro and in vivo. The SPC might influence AT2 lineage during the lung epithelium repair by activating signaling mechanism involving CD74 receptor.

Open Access
Article
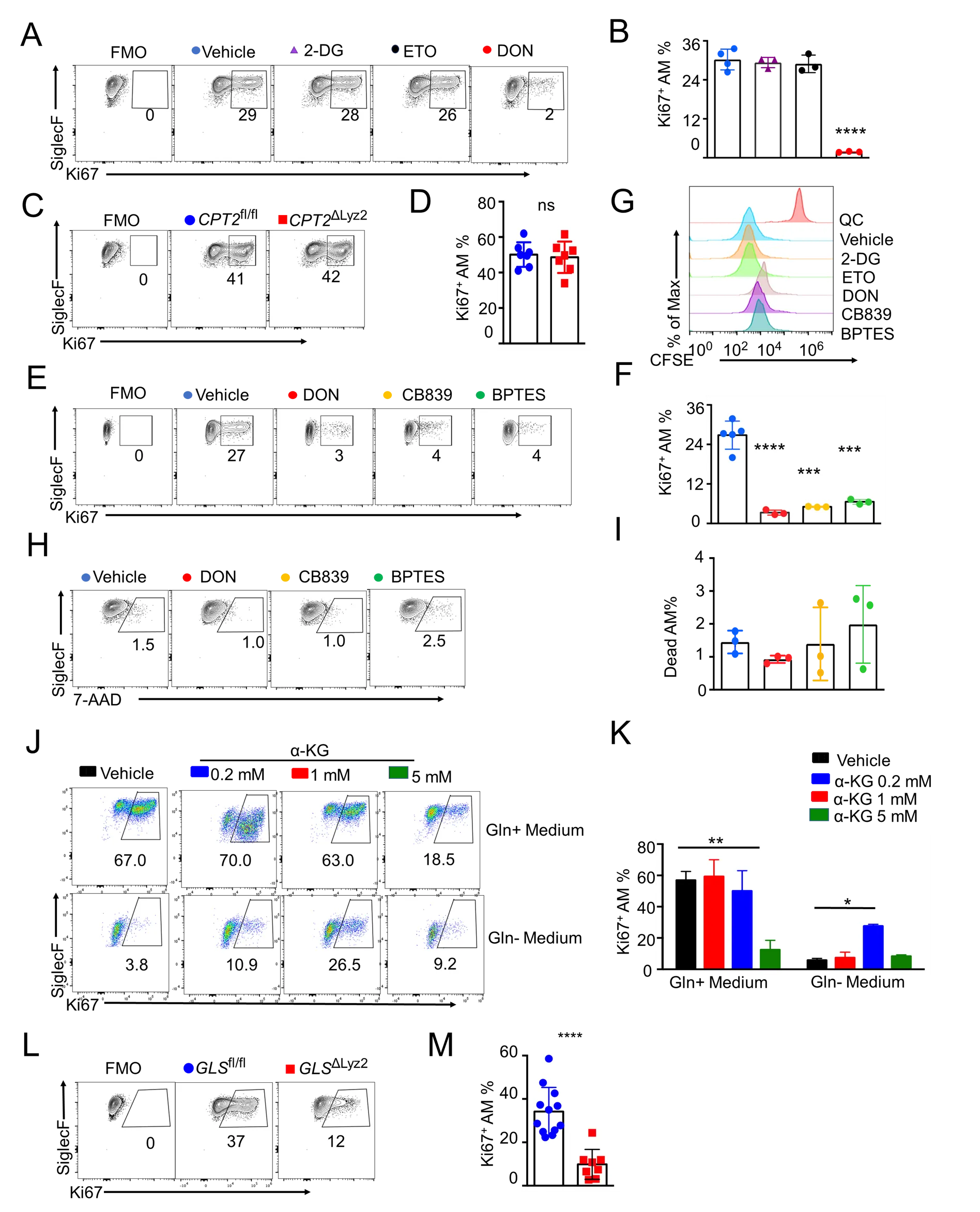
28 March 2024Glutamine Metabolism Is Required for Alveolar Macrophage Proliferation
Alveolar macrophages (AMs) are critical for normal lung homeostasis, surfactant metabolism, and host defense against various respiratory pathogens. Despite being terminally differentiated cells, AMs are able to proliferate and self-renew to maintain their compartment without the input of the hematopoietic system in the adulthood during homeostasis. However, the molecular and metabolic mechanisms modulating AM proliferative responses are still incompletely understood. Here we have investigated the metabolic regulation of AM proliferation and self-renewal. Inhibition of glucose uptake or fatty acid oxidation did not significantly impact AM proliferation. Rather, inhibition of the glutamine uptake and/or glutaminase activity impaired AM mitochondrial respiration and cellular proliferation in vitro and in vivo in response to growth factor stimulation. Furthermore, mice with a genetic deletion of glutaminase in macrophages showed decreased proliferation. Our data indicate that glutamine is a critical substrate for fueling mitochondrial metabolism that is required for AM proliferation. Overall, our study is expected to shed light on the AM maintenance and repopulation by glutamine during homeostasis and following acute respiratory viral infection.