Found 1 results
Open Access
Article
01 February 2024Molecular Regulation of Transforming Growth Factor-β1-induced Thioredoxin-interacting Protein Ubiquitination and Proteasomal Degradation in Lung Fibroblasts: Implication in Pulmonary Fibrosis
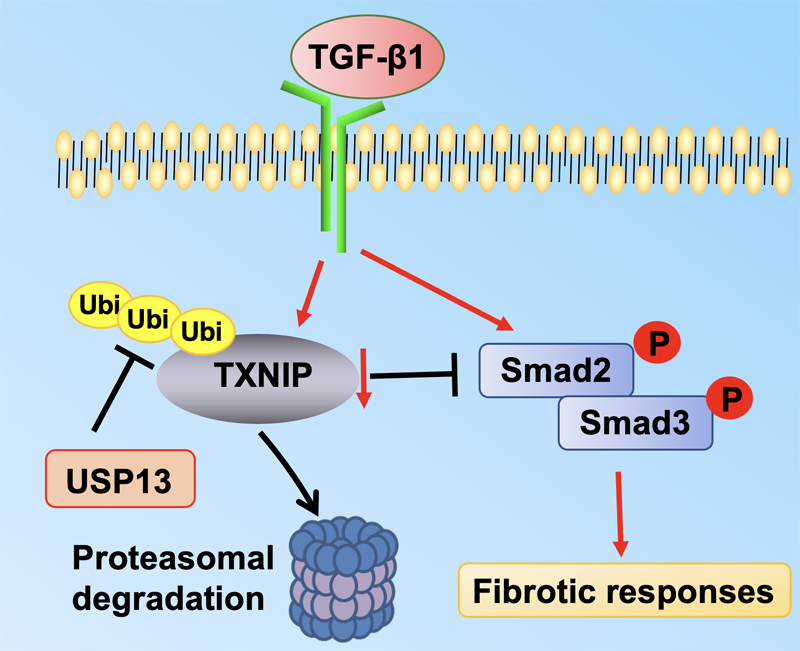
Thioredoxin-interacting protein (TXNIP) plays a critical role in regulation of cellular redox reactions and inflammatory responses by interacting with thioredoxin (TRX) or the inflammasome. The role of TXNIP in lung fibrosis and molecular regulation of its stability have not been well studied. Therefore, here we investigated the molecular regulation of TXNIP stability and its role in TGF-β1-mediated signaling in lung fibroblasts. TXNIP protein levels were significantly decreased in lung tissues from bleomycin-challenged mice. Overexpression of TXNIP attenuated transforming growth factor-β1 (TGF-β1)-induced phosphorylation of Smad2/3 and fibronectin expression in lung fibroblasts, suggesting that decrease in TXNIP may contribute to the pathogenesis of lung fibrosis. Further, we observed that TGF-β1 lowered TXNIP protein levels, while TXNIP mRNA levels were unaltered by TGF-β1 exposure. TGF-β1 induced TXNIP degradation via the ubiquitin-proteasome system. A serine residue mutant (TNXIP-S308A) was resistant to TGF-β1-induced degradation. Furthermore, downregulation of ubiquitin-specific protease-13 (USP13) promoted the TGF-β1-induced TXNIP ubiquitination and degradation. Mechanistic studies revealed that USP13 targeted and deubiquitinated TXNIP. The results of this study revealed that the decrease of TXNIP in lungs apparently contributes to the pathogenesis of pulmonary fibrosis and that USP13 can target TXNP for deubiquitination and regulate its stability.