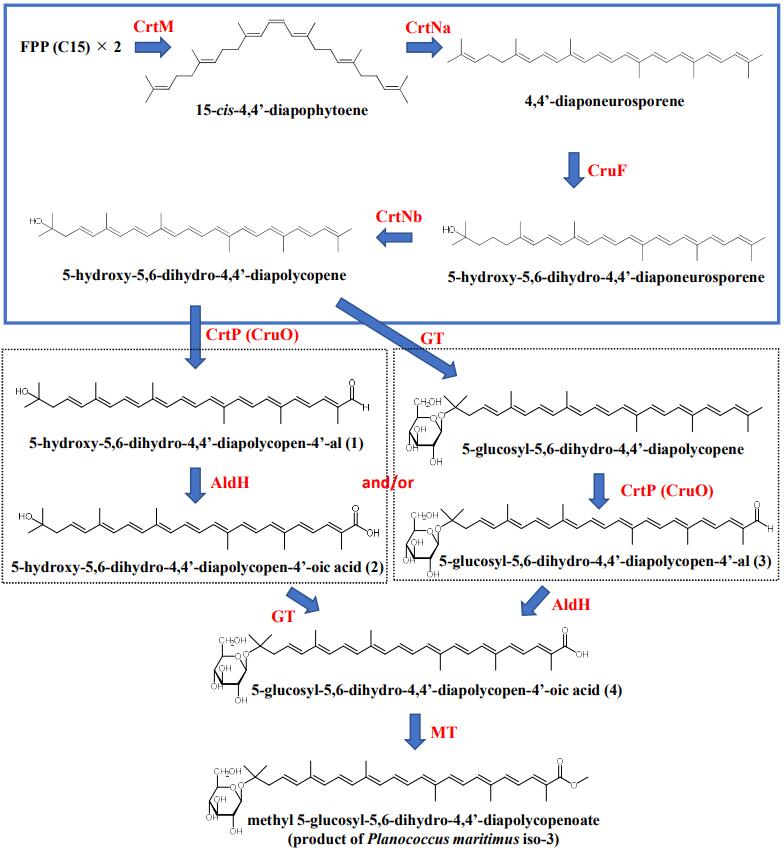
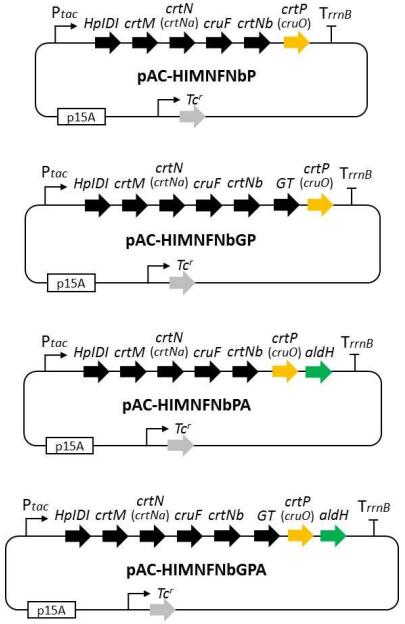
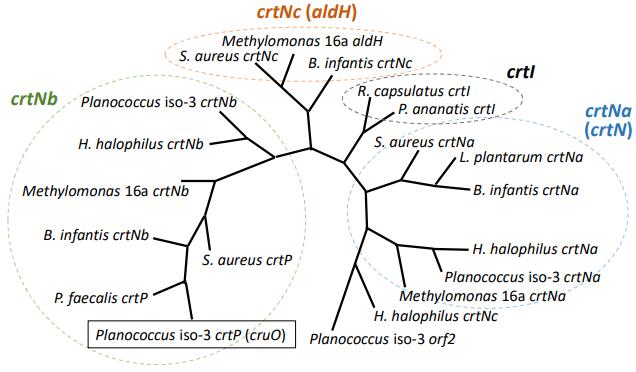
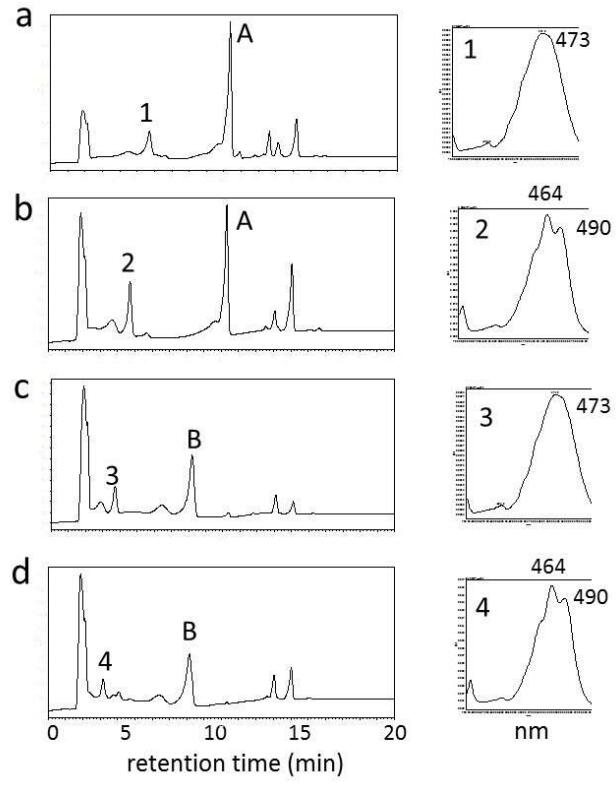
9th Asian Conference on Civil, Material and Environmental Science
The 4th Climate, Weather and Water Forum
International Conference of the German Society for Plant Sciences
Emerging concepts and Novel Mechanisms in Organ Fibrosis Workshop
ECB-IBS 2024
Five Editors-in-Chief Featured in the List of "Highly Cited
Prof. Yun Zhang, Scientific Advisory Board of /Cardiovascular Sci
Prof. Junhong Bai, Editorial Board Member of /Hydroecology and En
Five Editorial Board Members of /Photocatalysis: Research and Pot
5th Edition of Cardiology World Conference